Guidelines for pricing, positioning, and building consensus to gain your brand the best possible payer acceptance.
Almost every large drug company is looking at its late-stage pipeline opportunities and questioning whether it has the commercial potential to grow or even sustain global revenue levels over the next five years. Patent-expiration revenue cliffs and trickling R&D pipelines have left many companies with substantial revenue gaps. Meanwhile, global payers continue to tighten their reimbursement approval requirements and restrict the use of high-cost drugs to patients who benefit most. Moreover, the continuing economic recession has governments scrambling to implement austerity measures to balance budgets.
How can drug companies ensure success in this increasingly complex environment? How should they deal with payer-imposed market access hurdles and generate high prescription volumes within payer-endorsed treatment guidelines? How should drug companies organize themselves to guarantee that they are among the best-performing companies in the industry?
Here are some pricing- and market access-related guidelines that can help ensure strong and sustainable commercial performance.
TAKE THE PAYER PERSPECTIVE
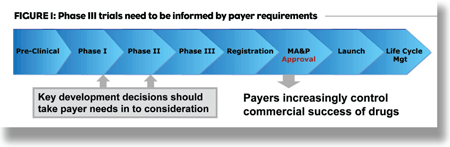
Incorporate the payer perspective in development decisions—or plan to underperform.
Now more than ever, the initial commercial success of new drugs is critical to their lifelines. To ensure favorable pricing and market access, manufacturers must have a firm grasp on the increasingly demanding and diverse payer environments across the world. Failure to do so will impair the company’s ability to survive the next round of competition among large and mid-size companies. The best-performing companies will not only understand payer motivations and requirements, but also systematically incorporate strategic pricing and market-access considerations into their drug development and commercialization decisions.
Payers worldwide are influencing physician-prescribing decisions in a bid to contain escalating drug costs and manage their budgets. Escalating healthcare costs and the recession have hindered the payers and governments’ abilities to raise and maintain adequate healthcare funds. Recent developments in the United States, Germany and the United Kingdom illustrate these challenges. Government price control continues to appear on the agenda of U.S. political discussions. In Germany, recent AMNOG (Arzneimittelmarktneuordnungsgesetz, the New Drug Market Order Law) legislation now dictates price control measures at a new drug’s launch. Further, while only cost-related utilization controls posed hurdles for particularly innovative new drugs before, the UK has recently announced value-based pricing.
While this is a compelling term, its meaning is unclear. It also prompts a question: why wasn’t value considered before? All things considered, a more physician-friendly value-based pricing system is likely to lead to significant increases in healthcare cost, which the government is unlikely to allow.
This changing environment makes it critical for pharmaceutical companies to take account of payer-specific requirements to obtain approval of drug price, reimbursement and unrestricted market access. This is particularly important since requirements for obtaining payer endorsement are both different from, and more challenging than, marketing authorization requirements established by the FDA, EMA or similar international regulatory agencies. For example, placebo-controlled trials (or trials designed to demonstrate non-inferiority to an approved drug) are often considered sufficient for a new drug to gain market authorization. However, payers are more likely to demand compelling evidence of important benefits over available therapies before they commit additional funding for new, and often more expensive, treatment options. This implies the need for statistically significant evidence of superiority in a direct comparison rather than a straightforward proof of non-inferiority. Payers also tend to put a stronger emphasis on long-term health outcomes rather than clinically accepted surrogate end points.
In summary: As payers tighten their belts, pharmaceutical companies need to be more aware of payer needs and payer system requirements to gain successful pricing and market access approvals. Misunderstanding the payer environment will likely be severely punished through commercial underperformance.
SHOW HOW THE PAYER BENEFITS
Provide evidence of meaningful and payer-relevant benefits over existing treatment options.
Obtaining payer endorsement of a drug requires an intimate understanding of what makes payers view an innovation positively and grant it favorable pricing and market access. To better understand these critical success factors, drug-makers must systematically evaluate both the relevance of benefits to payers as well as the robustness of the benefit claims (i.e., the strength of the evidence).
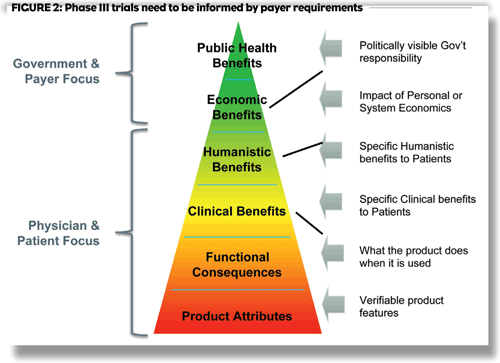
Many new drugs come with improvements, such as high potency, a unique mechanism of action, or a new delivery form. Payers do not see much value in these features unless they result in compelling clinical, humanistic, economic, or public health benefits. It is critically important to first understand which benefits payers value. Next, companies must gather strong evidence to support those benefits through their clinical and outcomes trial programs. Figure 2 illustrates a payer benefit pyramid that is useful in this context.
Clinical and humanistic benefits need to be strongly endorsed and valued by key clinical opinion leaders, since these experts most shape payer perspectives. Payers are increasingly critical of surrogate end points because they have not always correlated with compelling, long-term health outcomes benefits. Failure to demonstrate longterm survival for Vytorin, for example, has thrown a wrench into the use of low-density lipoprotein (LDL) as a surrogate end point for cholesterol-lowering agents. Similarly, clinicians continue to dispute the value of Progression- Free Survival (PFS) as a prognostic indicator of Overall Survival (OS) in cancer patients.
While economic benefits are very attractive to payers, it is important to link these claims to their underlying clinical improvement claim. Cost-savings claims (and their underlying assumptions) must withstand a critical evaluation. Even better, cost-savings should be an intuitively sensible consequence of a hard clinical claim evidenced in the trial program. Black box savings claims from models are rarely credible without a simple explanation of the clinical rationale for the savings.
When clinical benefits are sufficiently compelling, they can change the course of a disease and have a significant impact on public health. Examples of compelling public-health benefits are substantial survival increases for cancer patients, reduction in mortality of cardiovascular events and reduced transmission of HIV infections from mothers to their babies. Obviously, not every drug can create excitement with a clear and strong public health benefit, but these are the most compelling claims to persuade payers to cover a new drug or treatment.
Claims of benefits must always be accompanied by strong evidence. Clinically compelling data, for example, should be published in a reputable peer-reviewed journal or included in labeling. Claims referenced to data on file will rarely have any appeal for the payer community.
BUILD BROAD CONSENSUS
Facilitate cross-functional consensus building with structured analytical approaches.
Because market-access and pricing considerations often drive difficult and, at times, high-cost investment decisions at a pharmaceutical company, it is critical that the supporting analysis is both well understood and well supported by research and commercial disciplines.
Non-pricing professionals, however, often struggle to understand the complexities involved with many market access and pricing (MA&P) considerations. The environment is confusing, with many different payer systems around the world making relatively frequent changes. In addition, there are complicated tradeoffs to consider—between, for example, a rapid-launch strategy with limited claims and a delayed launch for a stronger value proposition. These tradeoffs require a structured analysis and comparison on the basis of a hypothesis-based revenue and net present value (NPV) analysis.
Again, payer requirements often differ fundamentally from FDA or EMA requirements, with payers seeking clear demonstration of superiority over a relatively inexpensive option before they consider granting an attractive price for reimbursement.
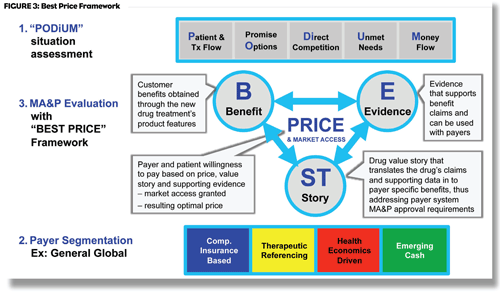
A helpful framework for analyzing a drug’s value proposition and ability to command favorable pricing and market access is the Best Price framework. This stepwise process examines benefits, evidence, value story and its impact on price and market access.1
Pricing and market access considerations will not always dictate the optimal development strategy. Other factors— such as investment needs, the probability of clinical success, and the time to market —will play a role. It is crucial, though, to make a proper evaluation of the tradeoffs based on a solid understanding of each option’s commercial implications.
AVOID PRICING GAFFES
Avoid profit-destroying pricing mistakes with well designed and executed research.
Clinical research that provides compelling evidence for a drug’s innovation and value requires significant investment. Pharmaceutical companies must clearly understand how payers will react to the resulting value proposition and related options. Payer and pricing research can help gather useful customer reactions and estimate payers’, and patients’, willingness to pay for a prescription drug; however, it is critical to avoid any response bias in these studies.
Researchers use a host of qualitative and quantitative methodologies to evaluate customer reactions to a drug’s value proposition and physicians’ willingness to prescribe or buy across a range of price levels. Many of these methods can result in response bias. Everybody wants a good deal, and (whether consciously or subconsciously) respondents tend to underestimate their willingness to pay when asked. Even methodologies—such as the venerable Gabor-Granger approach2—that are supposed to eliminate bias through randomization, can cause significant underestimation of optimal price. And although the Van Westendorp method3 is frequently used in drug pricing research, bias is practically guaranteed in its originally described quantitative approach.3 Yet over the years, we have seen many researchers continue to use it improperly without any acknowledgement of its inherent bias.
Establishing an optimal price for a prescription drug requires some critical steps:
- Ensure a good understanding of the decision-making process and interactions between key players. For example, physician and patient behavior related to co-pay or cost of a non-reimbursed drug.
- Introduce good research stimuli that appropriately represent the opportunity without over- or under-selling to the respondent.
- Properly design and execute research with an appropriate methodology and a well designed questionnaire.
Poorly designed payer and pricing research can easily result in substantial underestimation of willingness to pay. This, in turn, can significantly slash the innovation’s true return on investment.
COMMUNICATE
Improve communications with payers, patients and the public.
How can an industry that produces life-saving drugs have a poorer public reputation than the gun and tobacco industries, which produce products that kill? Communicating the value of individual drugs and the overall healthcare contribution of the pharmaceutical industry is vital for both the industry in its fight against intensifying government intervention and companies vying for acceptance of innovative, and at times costly, drug therapies.
During the emergence of HIV/AIDS, drug companies struggled to address the needs of the least developed countries. Providing drugs at lower prices to some of these countries has historically led to trade, price referencing and political demands for lower prices in developed nations. It took the industry a number of years to reconcile the conflicting demands of providing HIV/AIDS drugs at a socially acceptable lower cost to least developed countries and avoiding the negative consequences of international price differences. Unfortunately, the pharmaceutical industry never fully recovered from its public relations mistakes during this era.
Charging for health-related products or services is challenging, since companies seem to profit from what many perceive as a right. The drug industry’s cost structure is difficult to understand, and the industry’s claim that it needs to pay for new research has not been a satisfactory response for sick patients who need access to already available drugs. In light of this challenge, it is critical that pharmaceutical companies continue to demonstrate and communicate the value of new innovations brought to patients.
REFERENCES
1.The Best Price framework is fully described in “The Price of Global Health,” Gower Publishing; ISBN 9781409420521, 145-76. 2011. www.zsassociates.com/thepriceofglobalhealth/
2. The Gabor-Granger approach is fully described in “On the price of consciousness of consumers,” Applied Statistics, 10, 170-88. 1961.
3. The Van Westendorp approach is fully described in “NSS-Price Sensitivity Meter (PSM): A New Approach to Study Consumer Perception of Prices,” Proceedings of the 29th ESOMAR Congress, Amsterdam, 139-67. 1976.




