Rare diseases represent a major societal issue with significant costs. An estimated 400 million people are affected by more than 7,000 rare diseases worldwide.1 Patients, families, and caregivers often bear the brunt of costs directly and indirectly, whether it be medical costs for supportive or palliative care, available treatments, medical procedures, and hospitalizations or travel for medical visits, home modifications, and work or productivity loss. Historically, there has been a lack of shared responsibility for alleviating the burden of rare diseases by different stakeholders, including pharmaceutical companies, government bodies, policymakers, and society. But a societal approach will be needed to address this growing public health crisis.
Our team at Chiesi Global Rare Diseases, with support from IQVIA, set out to better understand the cost drivers contributing to the burden of rare diseases to determine the societal benefit of early diagnosis and treatment. Along with a number of recent publications on the societal and economic burden of rare diseases, our results2 found that the magnitude of the cost burden is staggering, but that it can be reduced with availability of new treatments. Results highlight the need to invest in pharmacologic innovation as well as diagnostics and newborn screening. Efforts to create improved healthcare policies to support people affected by rare diseases will be critical in the years ahead.
Building a Database of Priority Therapeutic Areas
We started by generating a database of 373 rare diseases covering approximately 8.4 million patients in the U.S. The rare diseases were selected following a review of more than 500 published articles and lists from sources including Orphanet, the Genetic and Rare Diseases Information Center, the National Organization for Rare Disorders (NORD), and the National Institutes of Health (NIH). The selections were discussed with experts at IQVIA, several patient advocacy groups such as the EveryLife Foundation for Rare Diseases, NORD, and Global Genes as well as therapy experts from 15 international institutions.
Further discussions with physicians and patient advocacy groups led to a selection of 24 rare diseases across five therapeutic areas—metabolic, hematologic, immunological, congenital, and neurological—that we reviewed in a detailed analysis. Together these 24 rare diseases impact approximately 584,000 people in the U.S.
 Evaluating Healthcare Costs
Evaluating Healthcare Costs
We assessed the cost of care associated with these 24 rare diseases and compared these costs with common mass market diseases such as diabetes, cardiovascular disease, Alzheimer’s disease, different types of cancer, and arthritis. The overall cost burden was evaluated across three categories: direct costs, indirect costs, and mortality costs. Compared to other similar studies, ours is unique in that we assessed mortality costs, which we defined as costs based on the value of statistical life (VSL), provided by the U.S. Department of Transportation,3 and the difference between average life expectancy4 and that for people with a rare disease.
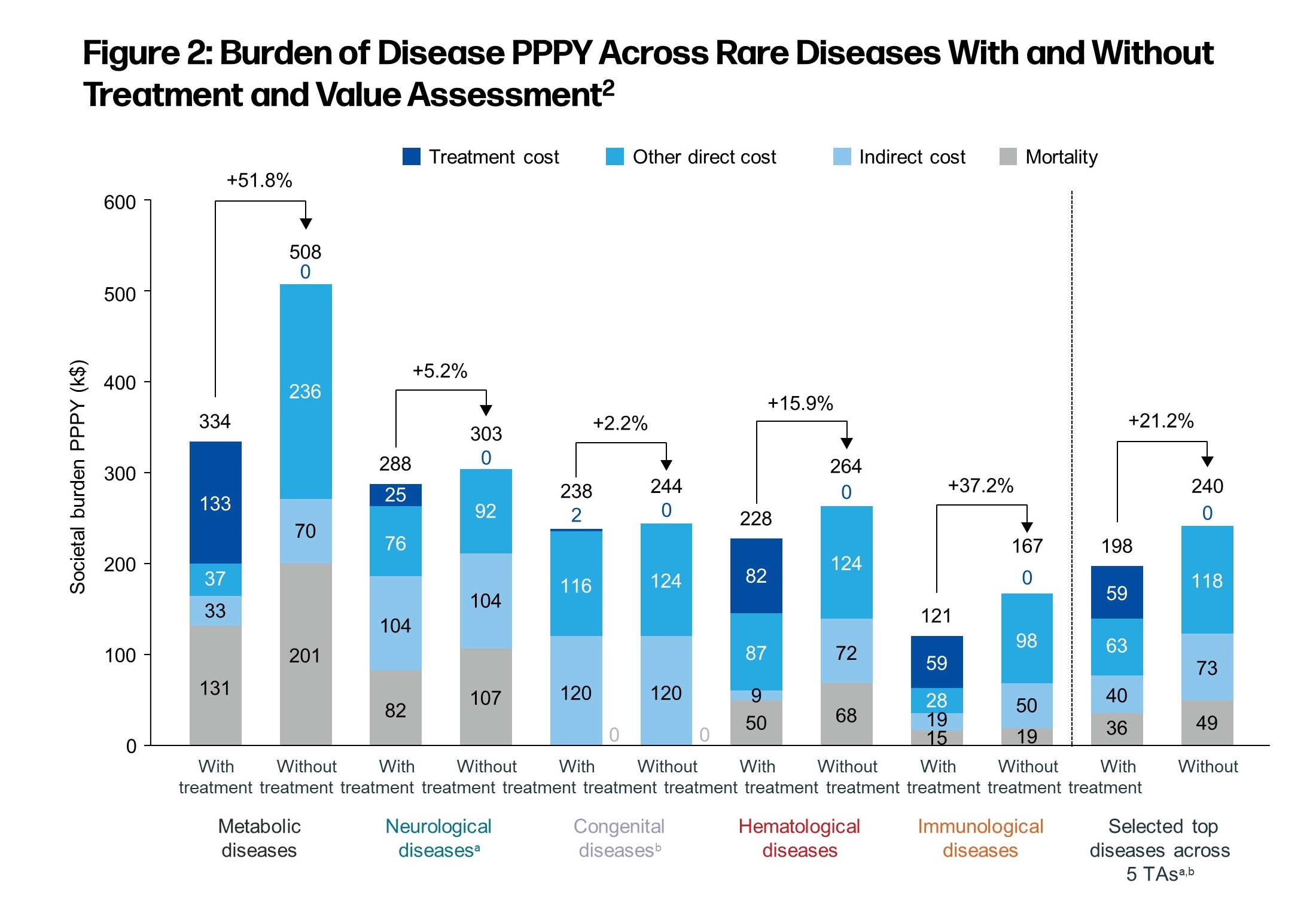
Our findings showed that for the 24 rare diseases reviewed in our analysis, the total cost to society is approximately $125 billion with an overall economic burden per patient per year (PPPY) ranging from $121,000 to $334,000, which is approximately 10x the cost associated with mass market diseases. The overall economic burden was generally driven by direct and mortality costs and was highest for metabolic ($334,000 PPPY) and neurological disorders ($317,000 PPPY). Indirect costs, while substantial, represented the smallest proportion of cost burden for rare diseases.
We also assessed the average cost when treatment is available versus when no treatment exists, which is the case for many rare diseases. One of the most interesting findings was that a lack of treatment was associated with about a 21% increase, on average, in total costs PPPY (ranging from a 2.2% increase for congenital diseases to a 51.8% increase for metabolic diseases). Also among the findings:
- Direct costs: $63,000 PPPY with treatment vs. $118,000 PPPY without treatment
- Indirect costs: $40,000 PPPY with treatment vs. $73,000 PPPY without treatment
- Mortality costs: $36,000 PPPY with treatment vs. $49,000 PPPY without treatment
Although most direct costs are covered by government and commercial insurers, substantial indirect and out of pocket expenses significantly impact patients and their families. But having access to treatment would effectively shift the burden relating to indirect and mortality costs into direct costs, which are more likely to be financed by private and public players including insurance companies, Medicare, and Medicaid programs. For example, with treatment access patients may no longer need to pay for indirect costs such as supportive or palliative care or shoulder costs such as productivity or work loss, as they and their caregivers may be able to return to work full or part time and generate income.
Private and public payers are often better positioned to handle substantial costs as they are generally well funded and can spread out potential risks over a broad patient portfolio including many insured and healthy customers, compared to patients and families impacted by rare diseases who are generally not affluent. By reducing the economic burden on patients and families, the shift in costs is likely productive and is shown to generate substantial value for society. Based on our analysis, the societal burden for all known rare diseases may range from $7.2 trillion to $8.6 trillion per year.
Impact on Future Healthcare Policies
A major strength of this study is that it presents an economic tool for analysis of the positive impact of rare disease treatments. These data can help justify increased governmental investment to ensure broader patient access to safe and effective therapies and policy proposals that reflect the unique challenges rare disease patients and companies often face.
Further investments should be encouraged by policymakers to foster and sustain innovation based on the positive economic return of rare disease therapies. The call for a shared responsibility framework is clear when considering that most states do not screen for all 35 newborn diseases in the Recommended Uniform Screening Panel (RUSP). Government actions that can help accelerate access to treatment include adapting a modern newborn screening system that provides equal access to a timely diagnosis for all infants.
This is vital because the Advisory Committee on Heritable Disorders in Newborns and Children current capacity is limited to adding two new diseases to the RUSP per year. The Senate must pass the Newborn Screening Saves Lives Act to strengthen screening systems and lead to broader diagnostic options.
Congress should also increase funding for the FDA’s Orphan Disease Grant Program, increase NIH rare disease research and funding, position rare disease clinicians and researchers to review Rare Disease applications and advise regulatory agencies, permanently reauthorize the Rare Pediatric Disease Priority Review Voucher Program, and preserve the Orphan Drug Tax Credit so that it continues to apply to multi-indications.
Funds for rare diseases should be allocated on par with mass market health conditions to reduce the associated significant societal burden. Social infrastructure must be adapted to increase caregiver resources and relieve families affected by rare diseases, as they bear especially high indirect or non-reimbursed expenses.
It is critical that pharmaceutical companies not only bring important new therapies to market, but also collaborate with government bodies, advocacy organizations, and other stakeholders to ensure policies increase, not decrease, patients’ access to therapies. At Chiesi, we are working with key stakeholders and other innovative companies and activists to build a shared responsibility framework so that more treatments can be delivered in less time, potentially reducing drug costs and improving access. This is how we are putting our pledge “health for all at all ages” into practice.
References:
1. Global Genes. RARE Disease Facts. Retrieved March 9, 2022, from https://globalgenes.org/rare-disease-facts.
2. Andreu, P., Karam, J., Child, C., Chiesi, G., & Cioffi, G. (2022). “The Burden of Rare Diseases: An Economic Evaluation” [White paper]. Chiesi Global Rare Diseases. https://chiesirarediseases.com/assets/pdf/chiesiglobalrarediseases.whitepaper-feb.-2022_production-proof.pdf.
3. Office of the Chief Economist. (2022, March 4). “Departmental Guidance on Valuation of a Statistical Life in Economic Analysis.” U.S. Department of Transportation. Retrieved 2021, from https://www.transportation.gov/office-policy/transportation-policy/revised-departmental-guidance-on-valuation-of-a-statistical-life-in-economic-analysis.
4. National Center for Health Statistics. (2022, January 13). “Life Expectancy.” Centers for Disease Control and Prevention. Retrieved 2021, from https://www.cdc.gov/nchs/fastats/life-expectancy.htm.








