Compared to other therapeutic classes, the asthma market is unique in that manufacturers can patent both molecules and delivery devices, allowing innovation to be achieved on two distinct fronts. In fact, some of the most widely-used asthma medicines today employ a molecule that has long since lost patent protection, instead relying on unique, patented delivery devices. An effective and easy-to-use delivery device is crucial for patients and physicians because it ensures that the appropriate amount of medicine is successfully reaching patients’ lungs.
According to the national Centers for Disease Control and Prevention (CDC), asthma is a common chronic “airway” disorder characterized by inflammation of the air passages that transport air from the nose and mouth to the lungs. In 2010, the CDC reported that 8.4% of Americans had been diagnosed with asthma, an all-time high and up from 7.3% in the year 2000.Children and blacks had higher rates of prevalence, including 9.5% of children and 11.2% of blacks. Asthma medicines are grouped into several categories according to treatment type, including quick-relief/rescue and long-term control.
Quick Relief/Rescue Inhalers
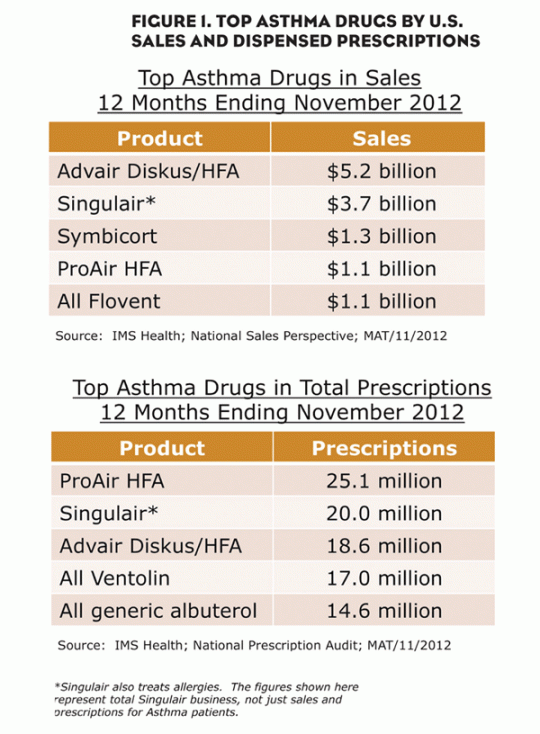
These medicines provide fast, short-term relief of asthma symptoms. The most popular active ingredient on the market today is albuterol, a short-acting beta-agonist (SABA). ProAir HFA, a branded albuterol sold by Teva Respiratory, leads the U.S. market in total prescriptions and is fourth in U.S. sales (Figure 1). In the 12 months ending November 2012, more than 25 million prescriptions were dispensed for ProAir HFA, and the product registered $1.1 billion in sales. Ventolin, another branded albuterol product, and generic albuterol, were also among the top asthma drugs prescribed during this period.
With most asthma patients requiring a rescue inhaler for emergencies, even when symptoms are controlled with other medications, the opportunity for ProAir HFA and other drugs like it is great. This is particularly true since asthma prevalence is increasing.
Long-Term Control Medications
These medications—including inhaled corticosteroids (ICS), long-acting beta agonists (LABA), leukotriene modifiers and combination inhalers—provide effective, long-term therapy for asthma sufferers. Patients who rely frequently on their quick relief medications should initiate long-term therapy.
Inhaled Corticosteroids
After progressing from rescue medication only, ICSs are often the first line of therapy for patients who need long-term control of their asthma. The most popular ICS is Flovent, which accounted for $1.1 billion in sales and had 6.75 million prescriptions in the 12 months ending November 2012.
Long-acting Beta Agonists
LABAs, such as Serevent and Foradil, open airways and reduce inflammation similar to SABAs, but their effect is much longer, typically 12 hours or more. In 2005, the FDA recommended the addition of a black box warning to these medications due to the results of a study that found an increased risk of death with use. These warnings were added in mid-2006 and LABAs remain on the market today as the benefits of these medications outweighed the risks. They are often used in combination with corticosteroids.
Leukotriene Modifiers
Leukotriene modifiers, which are taken orally, can be used alone or in conjunction with an ICS. A generic-version of the most popular leukotriene modifier, Singulair, was introduced in August 2012 and has seen immediate uptake—leaving Singulair with less than 5% of its typical prescription business after only three months.
Combination Inhalers
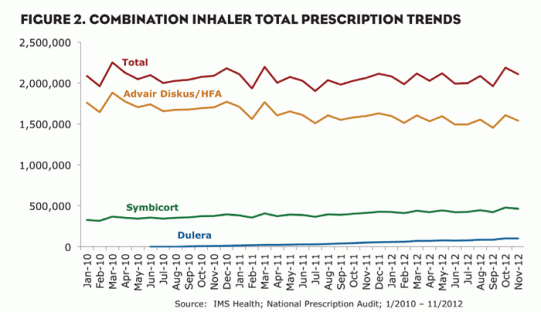
These products contain a corticosteroid and a LABA. They carry a black box warning due to their LABA component. There are currently three combination inhalers available: GSK’s Advair Diskus and Advair HFA, AstraZeneca’s Symbicort, and Merck’s Dulera. Total prescriptions (TRxs) for combination inhalers have been steady in recent years (Figure 2). In the 12 months ending November 2012, TRxs for these products increased just over 1% from the previous 12 month period.
Advair Diskus and Advair HFA, a fixed-dose combination of Flovent (fluticasone) and Serevent (salmeterol), have been extremely popular products for years and continue to lead the combination inhaler market. Labeled for asthma and chronic obstructive pulmonary disease (COPD), the Advair brand accounted for 75% of combination inhaler prescriptions in the 12 months ending November 2012. However, market share is declining, as Advair Diskus/HFA had accounted for about 80% of market TRxs over that same time period the previous year.
Symbicort, a fixed-dose combination of Pulmicort (budesonide) and Foradil (formoterol), has been gaining prescriptions since its introduction in mid-2007. Indicated for asthma and COPD, Symbicort yielded over 5.2 million prescriptions, representing 21% of the market’s TRxs, in the 12 months ending November 2012. Dulera, a fixed-dose combination of Asmanex (mometasone) and Foradil (formoterol), received FDA approval in June 2010 and was first dispensed by pharmacies in July 2010. Indicated only for asthma at this time, Dulera accounted for about 4% of market TRxs (931,000) in the 12 months ending November 2012.
Of all asthma products today, Advair, Dulera and Symbiocort are the most heavily promoted. In the 12 months ending November 2012, total promotional dollars spent include $704 million on Advair, $503 million on Dulera and $376 million on Symbicort. At a time when many brands are tightening their belts, GSK has increased spend in an effort to protect Advair’s business and stay front of mind with physicians as it prepares to introduce Breo, a new combination inhaler, with biopharma company Theravance. GSK and Theravance announced last September that the FDA had accepted their New Drug Application (NDA) for Breo, a combination of vilanterol and fluticasone (Flovent). While the initial NDA is for the treatment of COPD, GSK and Theravance have applied for an asthma indication in other regions, including Japan and the European Union.
Looking to the Future
As the industry looks to the future, this growing market—and the related COPD market—represent an opportunity for manufacturers to introduce novel molecules, new combinations and innovative delivery devices to help better treat patients. It’s expected that new combinations will be launched over the next few years that will further challenge Advair and other established brands.