Since its launch in 2000, ClinicalTrials.gov has steadily grown in size and currently hosts more than 260,450 research studies in 201 countries. Additionally, in the past 17 years, other non-government hosts of clinical trial data have also emerged—such as the World Health Organization’s (WHO) International Clinical Trials Registry Platform (ICTRP)—to further increase the ability of patients to find clinical trials and learn from their data. And over that time, legislation such as the Final Rule for Clinical Trials Registration and Results Information Submission passed with the goal to increase the transparency of clinical trials. And yet, despite all of that—and the best of intentions—the patients who use these registries struggle to actually find clinical trials that are right for them.
In fact, patients often find these registries to be uninformative—not because they don’t provide enough information, but because it is often too scientific and clinical for patients to understand. Patients also find it difficult and time-consuming to search these registries to find clinical trials that are relevant to them. Plus, these registries are often stuck in the digital stone age, without any mobile optimization or the ability to easily share the information they find.
“Many patients I interact with simply get intimidated or overwhelmed by trying to comprehend the language and understand the vast amount of information that’s available on these clinical trial registries,” says T.J. Sharpe, a two-time melanoma survivor who now serves as a writer, speaker, and patient advisor. “It just isn’t presented in a way that’s digestible so that patients can gain an understanding of what their different options are and how to weigh those different options against one another in to order to help make the best decision for their healthcare treatment.”
However, TransCelerate BioPharma recently presented a potentially better approach to what clinical trial registries might look like. The non-profit consortium of biopharmaceutical companies, which collaborates to find ways to improve the research and development process, released one of its latest concept papers: Improving the Value of Public Clinical Trial Registries to Patients: A Perspective and Call to Action.
The Initiative, Clinical Research Access & Information Exchange, from which these assets were created took a patient-centered approach to the design of future clinical trial registries. The workgroup included two patient members (one of which was Sharpe); a Patient Advisory Board (PAB), which was commissioned through the Center for Information and Study of Clinical Research Participation (CISCRP); a Site Advisory Group commissioned through the Society of Clinical Research Sites (SCRS) ; and representatives from TransCelerate’s Member Companies including AbbVie, Allergan, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, EMD Serono, GSK, Johnson & Johnson, Lilly, Merck, Roche, Pfizer, Sanofi, and UCB.
The Clinical Trial Registry of the Future
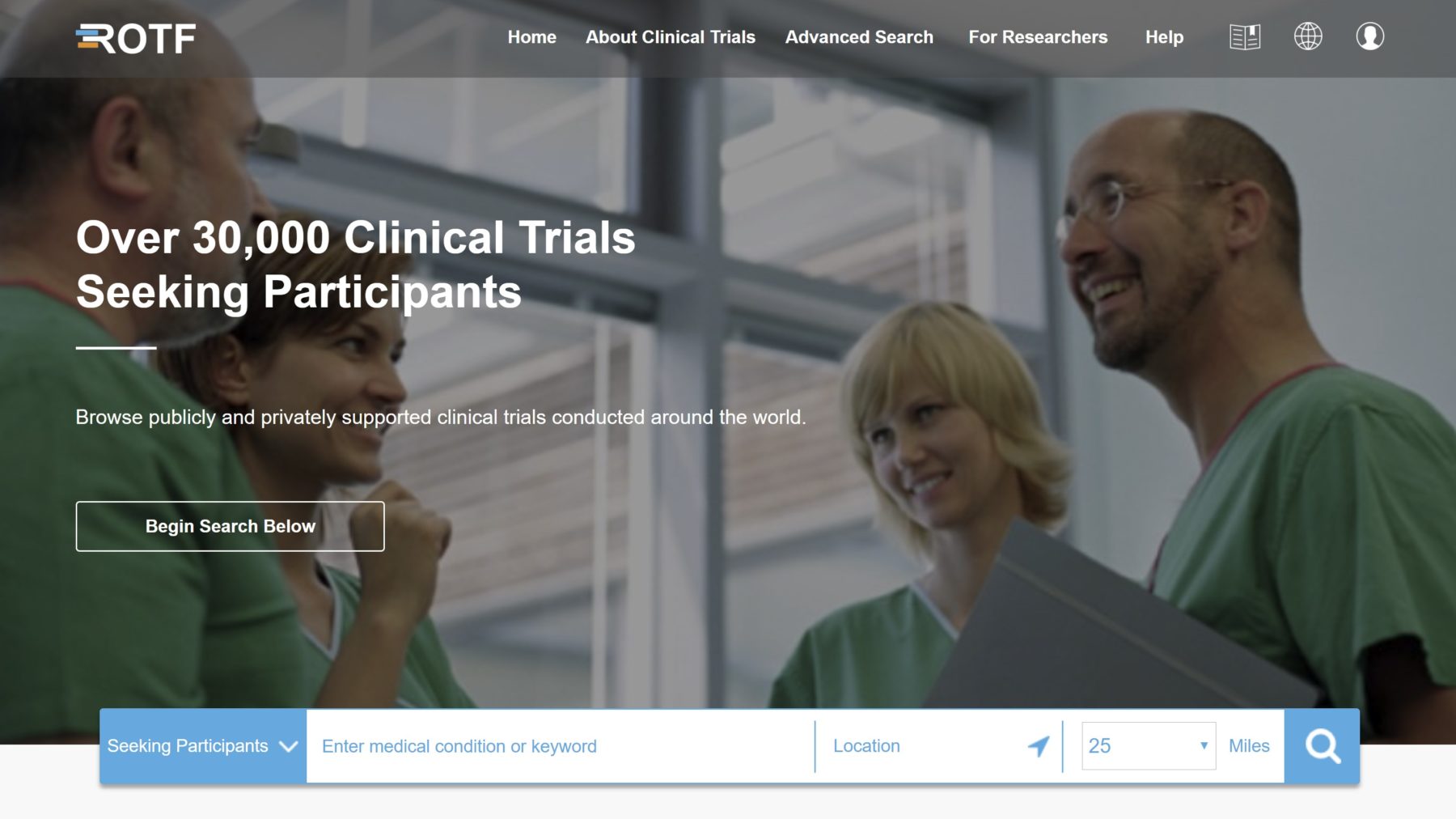
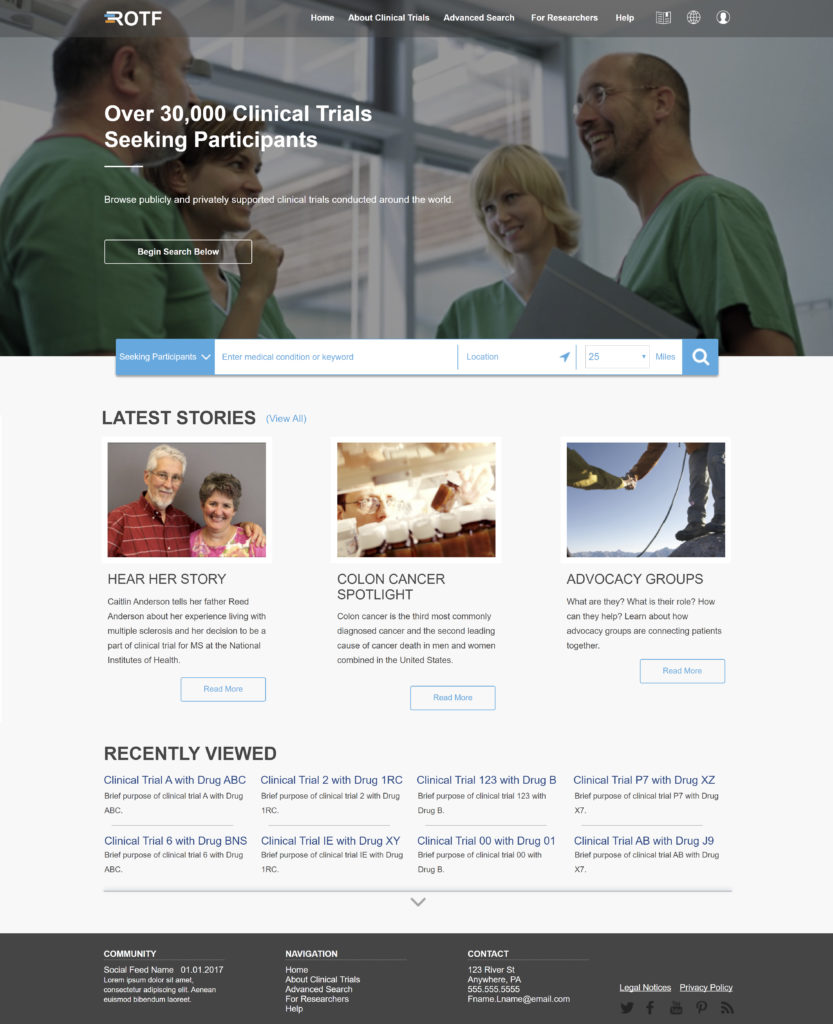
In addition to collaborating with the workgroup members, TransCelerate also conducted a survey, in partnership with CISCRP, of 3,000 people to better learn about their clinical trial information preferences. They also held virtual and in-person meetings with patients during which they literally asked people to draw what their journey is, and that could potentially include searching for a clinical trial. They also asked patients to work through different scenarios with a member of the workgroup to pinpoint where they were having problems when searching for clinical trials. All of this, and more, lead to creation of the Clinical Trial Registry of the Future concept and wireframe (seen below).
 “The key principles that came out of the initiative included: A clinical trial registry should be accessible, informative, and trustworthy in a manner that’s relevant to the people who are searching for clinical trials,” explains Roslyn F. Schneider, MD, MSc, FACP, FCCP, Global Patient Affairs Lead at Pfizer and External Engagement Lead for the Clinical Research Awareness Access and Information Exchange Initiative at TransCelerate. “And in keeping with those principles, patients wanted a welcoming site. Not cold and clinical, but personal and related to what they were putting in about themselves. Something simplified that would minimize the number of clicks and the amount of scrolling. And the other thing they made very clear: Even if they didn’t find a clinical trial option that potentially would be right for them, they wanted this experience to gracefully guide them to something that was an acceptable outcome, such as information that they could discuss with their doctor or an option to be re-contacted if something more suitable came up.”
“The key principles that came out of the initiative included: A clinical trial registry should be accessible, informative, and trustworthy in a manner that’s relevant to the people who are searching for clinical trials,” explains Roslyn F. Schneider, MD, MSc, FACP, FCCP, Global Patient Affairs Lead at Pfizer and External Engagement Lead for the Clinical Research Awareness Access and Information Exchange Initiative at TransCelerate. “And in keeping with those principles, patients wanted a welcoming site. Not cold and clinical, but personal and related to what they were putting in about themselves. Something simplified that would minimize the number of clicks and the amount of scrolling. And the other thing they made very clear: Even if they didn’t find a clinical trial option that potentially would be right for them, they wanted this experience to gracefully guide them to something that was an acceptable outcome, such as information that they could discuss with their doctor or an option to be re-contacted if something more suitable came up.”
All of these insights went into the design of the Clinical Trial Registry of the Future, a mock-up and functioning prototype that is meant to serve as a visual guide and discussion starter for healthcare teams or government registries thinking about making changes. The mock-up doesn’t actually include any live clinical trials, but examples to help those interested get a feel for the type of registries that patients would prefer to use.
The Features Patients Want from Clinical Trial Registries
“One of the things I suggested, that other patients wanted as well, was the ability to compare trials,” Sharpe says. “In real life, patients take notes on trials, which can involve printing out 10 different sheets of trials, highlighting the differences between each trial, and crossing out the all the similar exclusion criteria. Those are the manual processes that patients do. But this prototype offered a type of functionality in which you could search, for example, by a certain mutation type and then click to compare multiple trials. It is a very simple conceptual idea that made the user experience better.”
The simplified search function also included a basic search function available directly on the home page. Users can select one of three options (“seeking participants,” “completed with results,” or “all trials”), enter a medical condition (which features type-ahead functionality to help them find a condition faster), and their location (with the ability to automatically detect) along with a desired search radius from location.
As Sharpe mentioned, users can then filter results, but for more than just by a condition or mutation type. Other options include trial type, time commitment, age, gender, trial phase, and whether or not the trial includes placebos. The prototype even includes a “Help Me Refine My Results” option that takes users through a guided search experience based on their medical condition. And they could view their results on a Google Map to easily find the location of each trial.
Once users click to view a trial, the page features friendly, at-a-glance icons that provide basic attributes about the trial. Quick-action features are also located near the top of the page, including an “I’m Interested” button to quickly contact the nearest site location; a search bar to specifically search within that trial; and easy-to-click icons for printing or sharing the information via email, social media, etc. Additionally, by creating user accounts—which can be anonymous—users have the ability to bookmark content for a later time and set up alerts to be notified when there are new results to specific searches.
These are just a small sampling of the new features offered in the Clinical Trial Registry of the Future concept. You can learn more about the prototype at Clinical Research Access & Information Exchange Assets, which also includes findings from the research conducted about clinical trial registries.
“Now that this is open to the public, the next steps will be reviewing any public comments that we receive through the site,” Dr. Schneider explains. “Also, if anybody wants more information, we have people within the TransCelerate Initiative who would be happy to walk through the mock-up in a more detailed way. A lot went into creating this, and there is no question that however people would want to use it in a way that would be helpful for patients is something that we would support—even if the primary purpose was to start a conversation with the government-sponsored clinical trial registries.”
Sharpe adds, “When I speak to the industry, I let them know that the clinical trial experience can be what I call the Amazon or the Cadillac of medical care—it can be the best medical care that’s possible. The Clinical Trial Registry of the Future concept and wireframe can be a tool that will drive decision making, information sharing, and ultimately better healthcare for more people.”