In the evolving field of oncology drug development, clinical and financial insights can be developed using well-designed real-world evidence (RWE) studies. State-of-the-art modeling, data analytics, and simulation techniques designed to analyze various types of real-world data (RWD) collected during routine healthcare activities can create critical insights. Drug innovators designing new RWE research programs need to understand how payers perceive RWE and educate the market from an early stage of development.
RWE in 2021: Where Are We Now?
Today, RWE studies are used to assess how a given therapeutic option performs under real-world conditions and elucidate critical parameters such as effectiveness, safety, adverse events (AEs), drug-drug interactions, and tolerability. Similarly, RWE studies can be designed to enable comparative assessments of available therapies in each therapeutic indication. For oncology manufacturers, they must make RWE’s value clear to payers given patients’ limited time horizon.
RWE also supports oncology drug development, addresses significant unmet clinical needs, and can advance several important objectives:
- Indication expansion of an existing medication
- Improved dosing regimens
- Informing prevailing clinical treatment guidelines, such as the National Comprehensive Cancer Network (NCCN) guidelines
For payers, RWE-based findings can yield critical insights to inform reimbursement, pricing, and formulary decision-making. Such efforts can expand patient access to therapies, improve ways expenditures are allocated and prioritized, and drive revenue (and potential market share) for pharma companies whose products are shown to provide some clinical or commercial competitive advantage.
RWE may also impact payer perceptions to support innovative pricing and outcomes-based contracting strategies by proving the value of medicines to payers.
Recommendations for Oncology Product Manufacturers
Use of RWE data for approval processes is on the upswing, with data from Flatiron and other EHR sources becoming popular choices. However, to make the best use of the RWE:
- Enhance clinical trials: RWE can improve the initial design of clinical trials and help identify candidates during patient enrollment. Similarly, RWE-based studies, subject to regulatory approval, can help create a synthetic control arm based on clinical findings developed by studying available prescribing and healthcare data.
- Partner with regulators: Regulators are increasingly receptive to RWE that helps stakeholders understand the current standard of care, compare the findings from single-arm clinical trials, help expand long-term outcomes, and measure the effectiveness and safety of approved treatments across a broader cohort of real-world patients over time.
- Embrace technology: The development and validation of reliable data-analytics methodologies and the creation and analysis of curated data sets from EHR systems, diagnostic-testing laboratories, pharmacy and claims databases, disease and product registries, wearable devices, and more will require pharma to collaborate with internal and external experts.
- Encourage education and adaptation: Early and consistent engagement with key stakeholders is important.
How RWE Studies Can Yield Relevant Findings in Oncology
In oncology, clinical findings established using traditional Randomized Control Trial processes are inherently limited. Targeted therapies (approved for use in patients who demonstrate the presence or absence of a particular biomarker) are on the rise. However, clinical trials used to validate biomarker-directed therapies usually have a limited trial population.
While a small trial population may be sufficient for regulatory approval in certain oncology indications with high unmet clinical need, post-marketing RWE studies are essential to fill important knowledge gaps. Payers have noted the increasing importance of claims and registry data, as AEs form a large part of immuno-oncology therapies and impact overall budget and costs. Of note, oncology patients are especially looking for overall survival and tend to tolerate AEs.
RWE in the Real-World
The use of RWE has played a critical role in the approval process of several existing oncology therapies.
Using RWE to compare costs between first-line melanoma treatments: The study compared healthcare utilization—including hospitalizations, emergency room visits, and outpatient visits—and costs per patient per month (PPPM). Ipilimumab-containing regimens had the highest cost and resource use and pembrolizumab or nivolumab monotherapy had the lowest utilization and cost (Figure 1).1
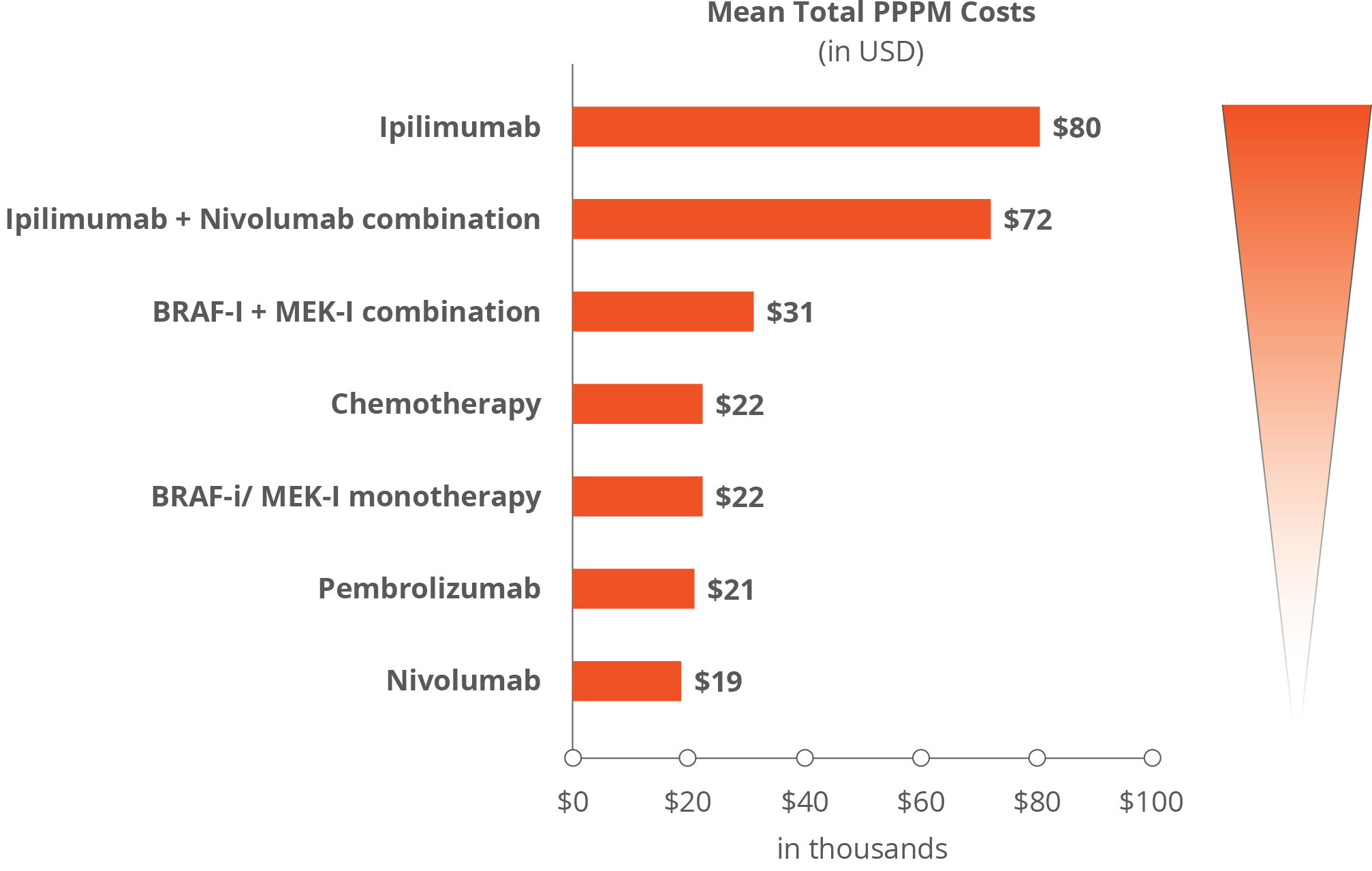 Based on RWE data, ipilimumab-containing therapies were found to carry higher rates of moderate to severe immune-related AEs. Consequently, pembrolizumab and nivolumab have been established as the preferred first-line treatment choice over ipilimumab.
Based on RWE data, ipilimumab-containing therapies were found to carry higher rates of moderate to severe immune-related AEs. Consequently, pembrolizumab and nivolumab have been established as the preferred first-line treatment choice over ipilimumab.
Post-marketing RWE study leads to regulatory approval in treating metastatic breast cancer in men: Ibrance is approved for the treatment of adults with hormone receptor positive (HR+), human epidermal growth factor receptive 2-negative (HER2) advanced or metastatic breast cancer, in combination with an aromatase inhibitor or fulvestrant. In April 2019—based solely on RWE findings—the FDA approved a supplemental New Drug Application for Ibrance that expands its approved use to address an unmet medical need—as the only CDK 4/6 inhibitor indicated in combination with an aromatase inhibitor for the first-line treatment of men with HR+, HER2- metastatic breast cancer in the U.S.2
This approval was based on EHR data and reports related to real-world, off-label use of Ibrance in male patients. Investigators demonstrated the safety profile for men treated with Ibrance is consistent with the safety profile for women treated with Ibrance, according to Pfizer.
RWE fills evidence gaps in post-approval settings for colon cancer treatments: In July 2021, the FDA approved Erbitux. This dosing change was supported by an RWE study of real-life experiences of patients with metastatic CRC. Eli Lily noted this approval marked a “foundational proof point for the successful use of RWD to fill evidence gaps in the post-approval setting for regulatory decision-making.”
The full promise of RWE in oncology is just beginning to be realized in terms of improving clinical outcomes and assessing the full cost impact and risk/benefit ratios for current and future lifesaving therapies.
References:









