Four categories of drugs that treat central nervous system (CNS) conditions—antiepileptics, antidepressants, antipsychotics, and neuropathic pain agents—share similar market dynamics: several mature agents and generics dominate the space, while payers seek to limit brand use and encourage use of low-cost generics.
With more brands losing patent protection, particularly in the antipsychotic category, companies with existing brands—as well as those about to enter the market—must understand how payers will respond to more generic entrants and how that will impact their access.
MANAGED CARE COMPLEXITIES CHALLENGE BRAND PLANNING
Managed care customers are interdependent and share overlapping roles, making it difficult for companies to identify the key influencers of brand access. In addition, regulatory, marketing, and disease-prevalence differences among payer types also impact brand-access decisions. Parsing these complexities is essential for companies building effective brand-access strategies.
Growing restrictions, combined with formulary tiers and myriad benefit designs, create a complex formula for determining access. This new definition of access takes into account:
1. Reimbursement, including copays, coinsurance, and benefit coverage
2. Restrictions, such as prior authorizations and step edits
3. Management, including patient compliance, guidelines, pay-for-performance, and disease management.
It is especially important for companies with new brands to understand this access paradigm, particularly what constitutes “preferred access.” Traditionally, companies sought second-tier, unrestricted access for their brands in exchange for appropriate financial concessions. However, the optimal-access strategy continues to evolve as market-leading generic alternatives emerge. At many plans, second-tier, unrestricted access is no longer a viable goal: companies must compete for third-tier, preferred status or second-tier status following a required trial of a generic.
CRITICAL MARKET EVENTS AFFECTING PAYER ACCESS
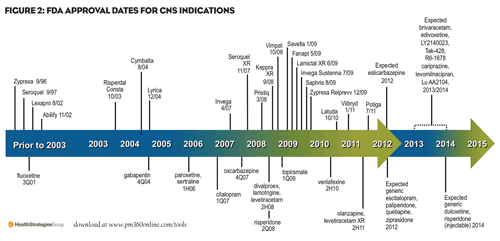
The CNS market is dynamic and evolving (see figure, “FDA Approval Dates for CNS Indications”). In 2011, several new agents gained approval, and more developments are expected in the coming years:
- Antidepressants. Forest’s Viibryd (vilozodone) received approval in 2011. In 2012, Forest’s Lexapro (escitalopram) will likely lose patent protection, a move that will drive payers to increase step therapy for remaining branded antidepressants. Two agents in phase III development for depression include Forest’s levomilnacipran and Lilly’s LY2216684. These agents must clinically differentiate themselves with payers to obtain optimal access.
- Antiepileptics. GlaxoSmithKline and Valeant’s Potiga (ezogabine) was approved in 2011 to treat epilepsy. Also last year, UCB’s Keppra XR became one of many generics, leaving Lamictal XR, Vimpat, and Lyrica to compete for optimal access at payers. Potiga will likely face difficulties in achieving access at plans that currently restrict access to antiepileptics. After receiving a complete response letter from the FDA in April 2010, Sunovion is likely to seek approval of an epilepsy indication for Stedesa (eslicarbazepine) in late 2012. In addition, UCB’s brivaracetam is in phase III development for the treatment of epilepsy and progressive myoclonic epilepsy type 1.
- Antipsychotics. In 2011, generic olanzapine (Lilly’s Zyprexa) joined generic risperidone as the two key generics in the antipsychotic market. Once AstraZeneca’s Seroquel (quetiapine fumarate) loses patent protection, it will create significant cost savings for payers, driving increased consideration to limit access to remaining brands. Forest’s cariprazine and Lilly’s LY2140023 are in phase III trials, and the companies are expected to file for approval in late 2012.
- Neuropathic pain agents. Lilly’s Cymbalta will likely lose patent protection in 2012, which will significantly impact access for Pfizer’s Lyrica for fibromyalgia and neuropathic pain.
EVOLVING PAYER MANAGEMENT
The market’s evolution raises new questions for CNS marketers, including:
- How do payers currently manage drugs?
- What are the key market, competitive, and strategic issues that affect payer access?
- How will payer management evolve in the next few years?
- How do health plans and PBMs influence access to, and use of, drugs in the category?
- How does brand access vary across Medicare, Medicaid, and commercial plans? Although plans recognize the need for access to CNS drug therapies, their cost-control imperative prevails. They are limiting utilization of costly brands through formulary management and pharmacy restrictions—though the degree of management priority and attention does vary by CNS category.
- Antidepressants. For most payers, antidepressants continue to be a high priority, due to the significant volume of prescriptions written each year and the associated costs. Following the patent expiration of two market leaders, Lexapro and Cymbalta, many plans will establish a generic-first step edit to limit access to remaining brands.
- Antipsychotics. These are also a top priority for many payers, particularly Medicaid plans, as five major brands will likely lose patent protection by the end of 2012. Despite historical sensitivities around limiting access to these important medications, plans are considering the use of restrictions on remaining brands to limit access. Multiple generic options address patient response variability issues, reducing physician and payer resistance to generic-first mandates.
- Antiepileptics. In contrast, managing antiepileptics is not a priority for any payer type, due to the sensitive nature of the disease and the fact that there is already high generic utilization. New agents entering this market will face few restrictions; however, they will have difficulty in obtaining preferential, tier-two access at most plans if they lack significant clinical differentiation.
- Neuropathic pain agents. In recent years, neuropathic pain has become a priority, particularly in Medicare Part D and Medicaid plans. There is widespread use of brand drugs in this category, even though multiple generics are available. Brand use will continue to grow through expanded indications, such as for treating fibromyalgia and chronic back pain, and will likely lead to increased payer management.
ACTIONS TO OPTIMIZE ACCESS
Once companies understand the complex factors that determine brand access in this important market, they can develop tailored, payer-access strategies to maximize revenue for existing and potential new-to-market brands. These may include:
- Contracting aggressively to avoid step edits, if this benefits brand success.
- Differentiating the brand. Payers seek head-to-head clinical trials and real-world data that demonstrate a better response rate than competitors.
- Educating payers on the complexities of the disease, and showing how your brand effectively treats specific populations better than competing brands and generics.
FIGURE 1: BRAND ACCESS EQUATION
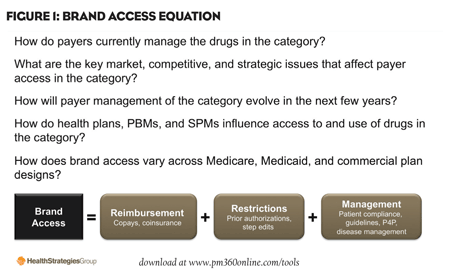
FIGURE 2: FDA APPROVAL DATES FOR CNS INDICATIONS
Download at www.pm360online.com/tools



