AT THE EADV CONGRESS
VIENNA (FRONTLINE MEDICAL NEWS) – The novel oral anticoagulants may provide effective adjunctive therapy in patients with calciphylaxis, Brian J. King, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a retrospective case series of 16 patients with a confirmed diagnosis of calciphylaxis who were treated with NOACs at the Mayo Clinic in Rochester, Minn., where he is a dermatology resident. The results were impressive, particularly given that the estimated 1-year survival following diagnosis of calciphylaxis is only 45%.
Nine of 16 patients responded to adjunctive NOAC therapy – most often apixaban (Eliquis) – with significant improvement in their cutaneous disease, defined as no new calciphylaxis lesions coupled with reduced surface area of lesions present at baseline. Four other patients experienced disease stabilization, while three had progressive disease marked by formation of new ulcerated calciphylaxis lesions and enlargement of the baseline lesions.
At a mean followup of 418 days, 9 of 16 patients were still alive. More remarkably, five of those nine experienced complete resolution of their clinical lesions and remained alive at a mean followup of 775 days.
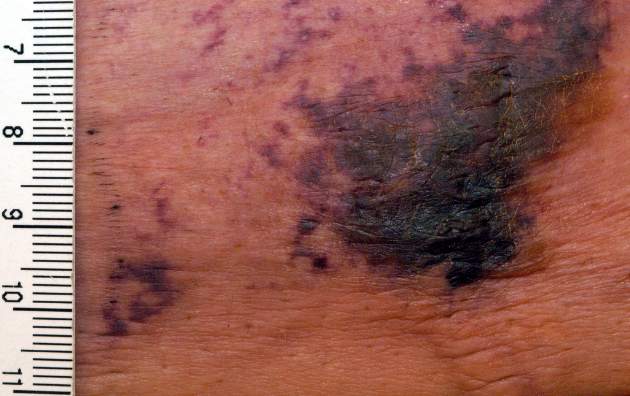
Calciphylaxis is a cutaneous manifestation of arteriolar thrombosis. It is classically associated with end-stage renal disease, hyperparathyroidism, a variety of hypercoagulable states, diabetes, and/or obesity. Fifteen of the 16 patients in the Mayo series were women. Fourteen patients had proximal involvement. The lesions occurred most often in fatty tissue on the hips, abdomen, thighs, breasts, and buttocks.
“It’s important to know that this is a deep, incredibly painful process and should not be confused with superficial crusted ulcerations,” Dr. King said.
A variety of treatments have been utilized for calciphylaxis, including sodium thiosulfate, debridement, advanced wound care, hyperbaric oxygen, and parathyroidectomy. But they are often ineffective.
Why not simply use warfarin instead of a costlier NOAC in addressing the problem? Because warfarin has actually been implicated as a cause of the vascular calcification that leads to thrombosis of dermal and pannicular arterioles. Indeed, 12 of the 16 patients in this series were on warfarin at the time of diagnosis of calciphylaxis, either for deep venous thrombosis, pulmonary embolism, or stroke prevention in atrial fibrillation. All were transitioned to a NOAC.
One group of Belgian investigators has provided evidence that strongly suggests the mechanism by which warfarin causes vascular calcification is via inhibition of vitamin K-dependent activation of matrix GLA 1, an enzyme which prevents calcification of vascular endothelial cells ( BMC Nephrol. 2014 Sep 4;15:145 ).
“It is possible and even likely that the vessel calcification we see in patients on warfarin predisposes to thrombosis,” according to Dr. King.
The pathologic diagnostic criteria for calciphylaxis utilized at the Mayo Clinic require skin biopsy evidence of medial calcification and intimal fibroplasia of pannicular arterioles with cutaneous necrosis. Extravascular calcium deposition or thrombosis of pannicular or dermal arterioles is also typically present.
The major clinical criteria are necrotic cutaneous ulcers over indurated plaques, or indurated plaques without ulceration in adipose-rich tissue. The minor criteria are livedo racemosa, hemorrhagic bullae, or hemorrhagic plaques.
Asked if the NOACs can be used interchangeably for treatment of calciphylaxis, Dr. King said the direct factor Xa inhibitor apixaban is the NOAC of choice for this condition at the Mayo Clinic because unlike rivaroxaban (Xarelto) it doesn’t require dosing adjustment in the setting of renal impairment, which is extremely common in patients with calciphylaxis. The direct thrombin inhibitor dabigatran (Pradaxa) is contraindicated in chronic renal failure. Edoxaban (Savaysa) is not on the Mayo Clinic’s formulary, but it is contraindicated in patients with a creatinine clearance of 95 mL/min or more.
Dr. King said he and his coinvestigators recognize that a retrospective case series such as this must be considered hypothesis-generating and nondefinitive. They have already begun a larger prospective comparative outcomes study.
Dr. King reported having no financial conflicts of interest.