There has long been discussion about the need to make written patient prescription medication information more user-friendly. Two-thirds of the over 65 population—who use a disproportionately larger amount of healthcare products and services—are unable to understand information given to them about their prescription medications.1 And, rates of low literacy are disproportionately high in the lower-income Americans eligible for publicly financed care through Medicaid.2 It is estimated that approximately one-third of the adult population in the U.S. has limited health literacy.3 Low literacy has been linked to poor health outcomes such as higher rates of hospitalization and less frequent use of preventive services with an estimated $106 to $238 billion dollar impact on the U.S. economy each year.4 So, as we look to incorporate greater numbers of these individuals into the insured healthcare system, the time is particularly ripe to agree upon and adopt a format to present more accessible and usable medication information.
Making it Happen
Catalina Health launched an eight-week quality improvement initiative, with other healthcare partners, in August of 2012, to disseminate newly-designed patient medication information (PMI) to patients filling prescriptions at participating pharmacies. The quality improvement initiative stemmed from an ongoing multi-stakeholder workgroup convened by the Engelberg Center for Health Care Reform at the Brookings Institution under a cooperative agreement with the U.S. FDA. The workgroup included: Catalina Health, the Medical Cognition Laboratory at Duke University, Emory University School of Medicine, the Feinberg School of Medicine at Northwestern University, GlaxoSmithKline, Janssen, Pfizer Inc., Purdue University College of Pharmacy and the Regenstrief Center for Healthcare Effectiveness Research. The FDA served as an observer. The workgroup was convened in March 2011 after workshops held by the Engelberg Center revealed that more evidence was needed to reform and develop a standardized PMI document delivered to patients when they pick up their prescriptions at the pharmacy.
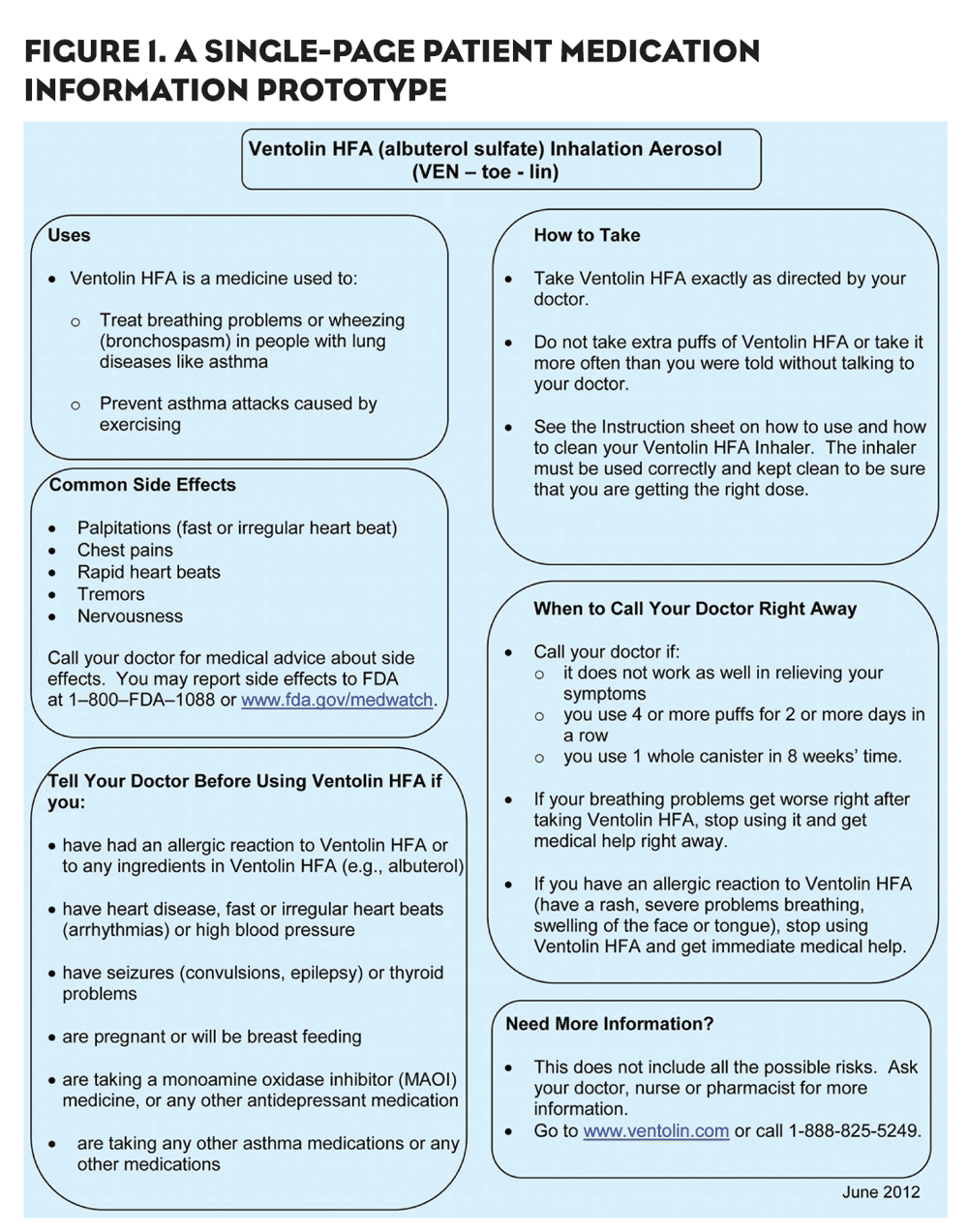
Members of the workgroup addressed needed reforms by developing a pilot PMI similar to one of the single-page prototypes that was developed by the FDA. Each document included a bold headline stating the name of the medication. Information presented in bulleted fashion was organized in boxes based on: Medication uses; important safety info; what to tell the doctor before taking the medication; how to take the medication; signs of and what to do in an emergency; common side effects; and phone numbers and websites for more info. See Figure 1 for an example.
Putting it to the Test
The new PMI was distributed to more than 32,000 patients in a leading pharmacy chain in California and Michigan for three brand medications in August and September 2012. Through voluntary telephone and online responses, patients were surveyed to confirm that they received the new PMI, to assess whether they found the info useful, and to determine how they would like to receive this newly-formatted patient medication information in the future.
The Results
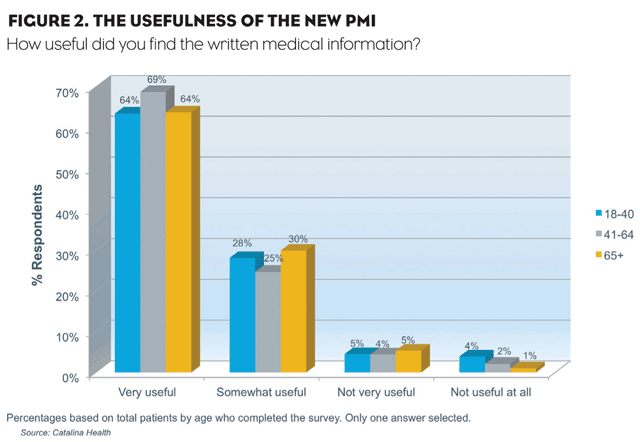
More than 90% of patients participating in the survey (3,255) recalled receiving the new patient information. Recall rate was the same for women and men and all age groups from 18 to over 65 had an equally high 90% recall rate of receipt of the single-page document. Additionally, more than 90% of all patients—both genders and all age groups—indicated they found the PMI useful (Figure 2). And two-thirds of recipients read the information and 40% kept it for future reference.
The high perceived value of a single document format is also supported by other proprietary research in which patients state a strong preference for information presented on one instead of multiple pages, for a well-organized layout in which information is presented in an “easy to extract” manner, and for use of larger fonts, more bulleted statements and less densely packed information. Similarly, research conducted with pharmacists reveals their preference for clear information on medication, side effects and dosing that can help facilitate discussion with patients.5
In addition to assessing readership and usefulness, the patient information quality improvement initiative also gauged preferences for non-print delivery vehicles. Patients under 65 were more likely to prefer digital options for communication of PMI, but 40% of the over 65 group indicated a preference for electronic delivery in addition to written. The vast majority of patients of all age groups indicated the desire to have a pharmacist explain the PMI along with receiving the written document, reflective of the high perceived value and level of trust consumers have in pharmacists.
Where to Go From Here
The patient information quality improvement initiative pilot program demonstrates that patients want, will read and will keep a single page of information about their prescriptions that is written in an easy to understand format, rather than multiple pages of complex medical text. Improving health literacy certainly requires much more than simplifying prescription medication information. Cultural, linguistic, educational and economic factors all must be addressed. But, progress usually takes place one step at a time. We look forward to the FDA considering these pilot results as they proceed with development of the PMI initiative.
References:
1. National Network of Libraries of Medicine, Health Literacy, http://nnlm.gov/outreach/consumer/hithlit.html.
2. M. Kutner et al. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. U.S. Department of Education, National Center for Education. Washington DC, 2006.
3. U.S. Department of Health and Human Services, http://www.nlm.nih.gov/medlineplus/healthliteracy.html.
4. Vernon, John A., et al, University of Connecticut, Department of Finance. Low Health Literacy: Implications for National Health Policy, 2007.
5. Proprietary qualitative and quantitative patient and pharmacist market research commissioned by Catalina Health, conducted by Double Helix Development Corporation, 2010–2013.