The growth of EHR adoption in the United States will give researchers access to a new source of data that offers several advantages, including the real-world tracking of how drugs are being used.
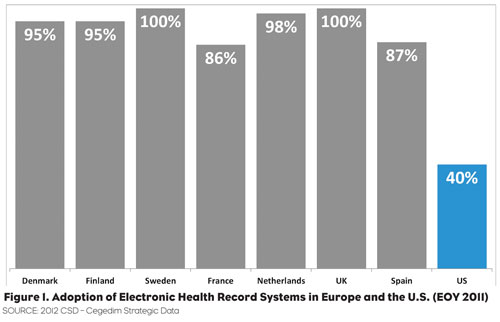
Over the last 20 years, pharmaceutical marketing research in the U.S. has grown into a fully realized science due to the wealth of data sources that became available with the increasing computerization of patient treatment information—initially from prescription data and later from other sources such as insurance claims. Over this same period, Western Europe developed similar capabilities based on the almost universal adoption of Electronic Health Records (EHRs) to support the care optimization and cost containment needed in their Universal Care systems (Figure 1).
The passing of the American Reinvestment and Recovery Act accelerated EHR adoption in the U.S., with over 40 percent of U.S. medical practices now using computerized records—reaching over 75 percent of the practices with more than 25 doctors. This spreading adoption brings to the table a new source for market- ing data in the U.S.—one that offers several advantages over the existing retail and claims-based systems.
What’s Different about ehr Data?
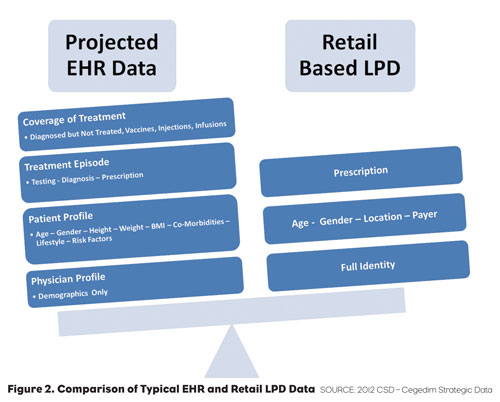
If we compare a typical EHR record to data found in a typical prescription record created from retail-based Longitudinal Patient Data (LPD), we find some overlap in the data but many elements that are unique to chart-based data1 (Figure 2).
One of the key advantages to the use of EHRs is that they represent a complete and integrated real world data source. Researchers in the past have relied on synthesis of diagnosis from retail LPD data or have com- missioned Primary Market Research (PMR) studies to observe trends from small samples of patient records such as chart reviews. With EHR data, these researchers can now do real-world tracking of incidence, prevalence and treatment with sample sizes sometimes exceeding a million patient records. EHR data brings significantly higher fidelity to research (both for marketing and medical studies) than other non-chart based data sources.
Privacy Considerations And Data Availability
One area that has a huge impact on the use of any patient data is patient and physician privacy regulations. EHR data is de-identified but, in Eu- rope, individual countries still place significant restrictions on the use of the data. One major challenge with extracting data for marketing research from European patient data is that, unlike the uniform privacy restrictions in the U.S., each nation has differing regulations on use of the data. This includes aggregation of physician identities and additional restrictions on patient information. In Italy, for instance, no data can be provided on patients under the age of 18.
HIPAA and other U.S. privacy laws provide more consistent privacy regulations than the layered “EU plus individual country” regulations that apply to European data. While individual U.S. states are enacting additional regulation on sales interactions, most marketing research has not been impacted by these regulations.
Another factor in data consistency is patient tracking across the healthcare system. In Europe, most EHR systems were developed to meet the record-keeping needs of individual doctors and to enable aggregated tracking of healthcare costs in single-payer systems; there was no thought to the development of Accountable Care Organizations (ACOs) or patient tracking across the entire system. For that, and other reasons, most European EHR systems can’t track patients be- tween generalists and specialists. Oftentimes, GPs do record diagnoses from treatment initiated by the specialists but those records can’t be relied on for seamless patient tracking outside the GP’s care.
In the Patient Protection and Affordable Care Act, ACOs take up several pages and are positioned as a critical part of America’s 21st century healthcare system. ACO management requires integrated record-keeping and, for the ACO patients at least, will mean EHR data that will be able to track the full spectrum of care provided for a patient.
EHR DATA ALLOWS THE RESEARCHER TO UNDERSTAND WHY
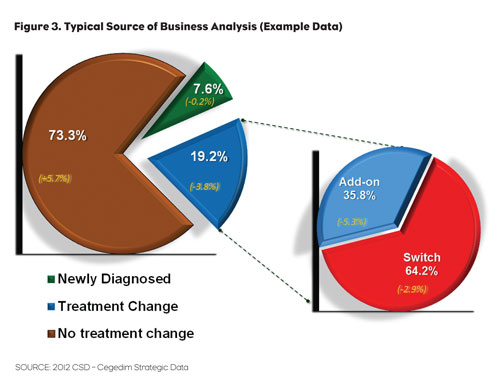
One of the big differences between retail and EHR data is that it allows the researcher not just to address the “what” of market dynamics but to also see why trends are occurring. See Figure 3 for a typical Source of Business diagram that we might see from retail LPD.
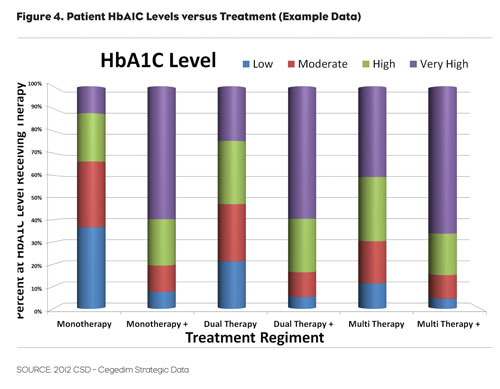
Longitudinal tracking based on LPD allows us to move beyond this to see the why. In Figure 4, we are able to track market share of the leading diabetes treatments relative to patients’ HbA1C levels.
EHR DATA ALSO SHOWS THE HOW
Another typical use for EHR based data is the analysis of drug posology—how a drug is administered.
For example, in a diabetes treatment with insulin there are a wide variety of methods for the administration of insulin: syringe from a vial or pre- filled, auto-injecting pens, or insulin pumps. Patients can take a long- acting insulin once or twice a day or they could use a long-acting product in conjunction with a short-acting “bolus” that is given in conjunction with meals or as is needed based on the patient’s readings from their blood glucose meter.
Understanding whether a drug is used as first-line, second-line or tertiary therapy as well as knowing whether it’s prescribed for all patients or just for the sickest patients can have a great impact on marketing. This is especially true as new introductions are being planned when the ability to understand and quantify the unmet needs of the market is invaluable.
Also critical to the business development and marketing research functions is the ability to understand how drugs are actually utilized in “real world” scenarios (including off-label use). Companies have used data sources such as insurance claims as proxies for diagnosis in the past— sometimes combined with retail prescribing data. While expedient, several factors complicate the use of diagnosis codes from claims data. EHR data, since it is sourced directly from the chart, zeros in on diagnosis and treatment.
DISEASE INCIDENCE AND PREVALENCE
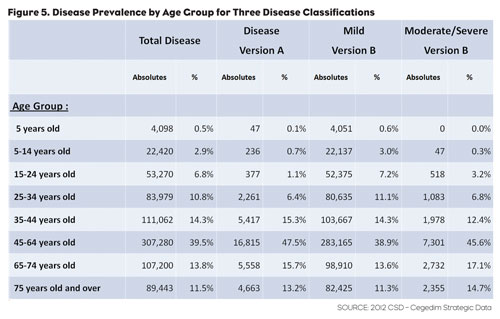
Analysis of disease incidence and prevalence usually either requires a PMR study or forces the researcher to rely on reporting from public health authorities. Both options normally result in low resolution results since a significant investment is typically required to increase the sample size enough to achieve a high degree of accuracy. Since EHR data allows us to cross patient demographics with exact diagnoses while offering large sample sizes, it gives the researcher the ability to easily drill down to the needed level of detail for the analysis being performed (Figure 5).
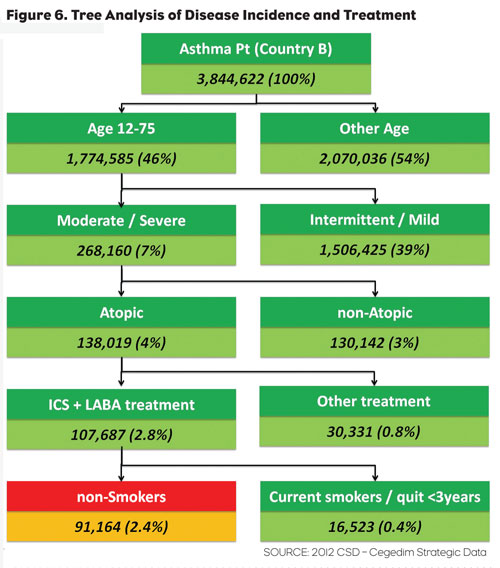
Being able to visualize the impact of a disease on a specific population brings significant clarity to understanding unmet needs and disease impact on defined populations. In our final chart (Figure 6), we see the breakdown of asthma treatment as applied to smokers and non-smokers within a defined age and disease classification. Being able to combine this rich set of patient demographics, diagnosis and treatment data is one of the hallmarks of EHR-based research.
ADOPTION OF EHR DATA FOR MARKETING RESEARCH
The growing introduction of EHR systems to the United States and the forthcoming availability of EHR data will probably be more evolutionary in its impact on Pharmaceutical Management Science than the revolutionary impact of the introduction of retail Rx data. The model will likely more closely follow the introduction and slow growth in the use of retail- based LPD as a tool for the market researcher. EHR data represents a significant breakthrough for those researchers who need high-precision or to understand combined disease and treatment information, however, for most researchers EHR data will simply bring more accuracy to their existing work.
The two key points to keep in mind about EHR data is the linking of disease to treatment and the ability of the researcher to perform longitudinal monitoring of testing, diagnosis and treatment. No other data source can as easily or as economically fulfill these two roles.
1. EHR data is used for both marketing research and medical research. Medical researchers often make use of different data elements or raw (un-projected) data. Marketing data is normally projected.