from blood
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) ( Blood. 2016;127[9]:1102-8 ).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
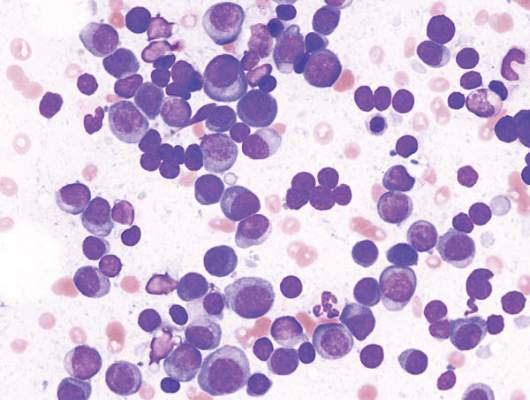
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).