Copay coupon programs are under attack. Class action lawsuits filed in March are charging that copay coupons constitute illegal bribes made by drug manufacturers to patients. Consulting and business intelligence firms weigh in on the case with a closer examination of some of the claims being made.
In March, several health plans in Community Catalyst’s Prescription Access Litigation (PAL) coalition filed class action lawsuits charging that copay coupons constitute illegal bribes made by drug manufacturers to patients. The lawsuits were filed by the American Federation of State County and Municipal Employees (AFSCME) District Council 37 Health & Security Plan Trust, Sergeants Benevolent Association, the New England Carpenters, and the Plumbers and Pipefitters Local 572 Health and Welfare Fund in four federal courts (New York, Chicago, Philadelphia, and Newark) against nine drug manufacturers (Abbott, Amgen, AstraZeneca, Bristol-Meyers- Squibb, GlaxoSmithKline, Merck, Novartis, Otsuka America Pharmaceutical, and Pfizer) and cited 25 brand-name drugs (including Lipitor, Nexium, Crestor, Diovan, Enbrel, Humira, Abilify, Nasonex, Vytorin, and Lovaza).
The suits allege that copay coupon programs undermine the insurers’ relationships with their beneficiaries, constitute insurance fraud, and violate the Racketeer Influenced and Corrupt Organizations Act (RICO) as well as anti-trust laws.
It is important to note that these cases do not necessarily attack every copay coupon program and that the plaintiffs named the drugs for a specific reason.
“Our purpose for naming these particular drugs in the ligation was that these are all drugs that have far less expensive generic alternatives and the coupons are used to promote the drugs over those alternatives,” said PAL director Wells Wilkinson.
The 130-member PAL coalition is certainly no stranger to lawsuits against the pharmaceutical industry. They were involved in the Average Wholesale Price lawsuit and in suits charging deceptive advertising for products such as Vioxx and Celebrex.
According to Wilkinson, the PAL started to voice concerns over copay programs back in 2005. Back then, they focused on the potential for coupons to deceive consumers and distract them from the safety and cost-benefit analyses that should go into the taking of any drug. More recently, a November report from the Pharmaceutical Care Management Association (the PBM trade organization) highlighted the long-term costs of copay programs. The report estimated that coupon programs will increase drug costs by $32 billion nationwide by 2021. The numbers alleged by PCMA played a significant role in alerting the health plans and other payers overall about how significantly coupon use to market drug products has grown and was continuing to grow.
Now the PAL is focused on preventing drug companies from using copay coupons to promote expensive brand-name drugs as first-line treatment options when there are generic alternatives available.
A LOOK AT THE NUMBERS
Consulting and business intelligence firms that have studied copay coupons for the pharma industry say that the numbers tell a different story.
“I don’t know why they picked [these drugs] but they certainly aren’t the ones to back the claims they are making,” said Mason Tenaglia, Managing Director of The Amundsen Group. “They basically picked the largest, biggest, deepest-pocketed pharmaceutical companies in the U.S. and focused on them.”
The Amundsen Group, a strategy and analytics consulting firm, compiled brand-by-brand and class-by-class copay coupon data from longitudinal patient data received from Wolters Kluwer as well as the redemption data their clients received from copay offset program vendors. The transaction-level data show when and where copay cards are used, and track usage of branded and generic drugs, as well as switches among them. Data for a single therapeutic class includes somewhere between 20 to 30 million transactions.
The Amundsen Group found that copay coupon programs are not correlated with lower generic uptake in any of the major therapeutic classes. The analysis also revealed that, in most cases, coupons are used for Tier 2 brands and for contracted prescriptions, in which the payer has already negotiated a rebate from the manufacturer.
“Without longitudinal data and without the knowledge of secondary payers, there is no way you can draw the conclusions that PCMA has drawn. And I am sure they didn’t use these types of data in [their report],” Tenaglia said.
Wilkinson pointed to a recent survey conducted by the National Coalition on Health Care, Seniors’ Awareness and Use of Prescription Co-pay Coupons in Medicare, as evidence that these coupons do decrease generic uptake. Federal programs like Medicare bar coupon cards, but still 6% of seniors said they have used a coupon card and 4% have switched from a generic drug to a brand-name prescription medication because of a copay coupon.
“I would not be surprised if that pattern holds true for the rest of the population using these copay coupons,” Wilkinson added.
Meanwhile, the Zitter Group (ZG)—a business intelligence company that works with life science companies on issues relating to access, reimbursement, and managed care— found another pattern among the numbers. ZG performed an exhaustive search of the internet and contacted hundreds of different brands to see if they had copay programs. So far, ZG has discovered 484 currently available copay programs. Of those, only 3% are sponsored by brands (such as Lipitor) that have a direct generic equivalent. Another 10% or so have indirect competition, such as non-chemically equivalent generics in the same therapeutic category.
Furthermore, according to ZG’s, Co- Pay Offset Monitor 2012, only five brands had programs that reduced copay to $4 (while one other reduced the co-pay to $20). Also, 13% of the programs covered biologics, among which only four brands—all growth hormones—had some kind of generic competition.
“I think one of the main problems I have with the whole discussion is it only focuses on a very narrow piece of the whole thing,” said Mark Zitter, ZG’s founder and chief executive. “And you could have a very robust discussion about that narrow piece; just realize that’s a very tiny piece of what’s going on.”
Wilkinson admits that he has found similar results in his own study of the programs. Of the 360 programs he found, about 8% involved drugs that are or will be available as a generic in the next year-and-a-half. In fact, of the 25 brand-name drugs named in the ligation, only Lipitor currently faces competition from a chemically equivalent generic.
“In most cases, that’s not what’s happening,” Wilkinson said. “It’s really promotion of expensive brand-name drugs that’s competing with two or three or more generic alternatives in the same therapeutic class, but they are not chemically identical to the brand-name drug.”
Tenaglia also acknowledges that Lipitor, for the most part, is the exception. But, he says, an important point is being ignored.
“Branded Lipitor is likely more cost effective for payers than the generic alternative by Teva, Ranbaxy, and Watson,” Tenaglia said. “Otherwise they wouldn’t do it. Do you think the people at United aren’t very smart about what statins will cost them net of rebates? This is also why branded drugs stay on the Medicaid formularies long after they go generic, because the rebates that have been mandated by the best price plus CPI penalty are so significant, often 70-90%, that it’s in the payer’s (in this case the state government’s) best interest to keep the brand on formulary in Medicaid. It’s cheaper than paying for the generic alternatives.”
Tenaglia also questions some of the other drugs named in this case. “There are no generic alternatives to Humira and Enbrel, and their copay cards are critical to keeping patients adhering on their products,” he said.
The potential ramifications of the case are still unknown; especially the issue of whether all copay coupon programs could be in jeopardy.
“I think ultimately it’s going to be up to a judge to decide whether a ruling in this case is going to broadly apply to all drugs that are subsidized in this way,” Wilkinson said.
Physicians’ Perspective on Copay Programs
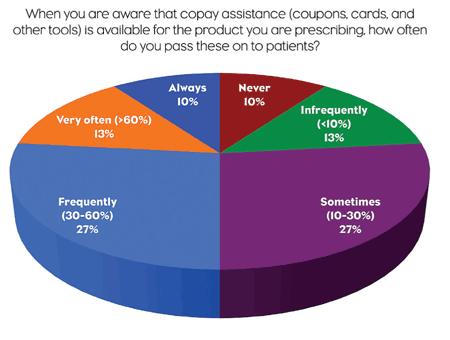
At the end of April, PM360 and WorldOne Interactive conducted a MedLIVE poll of 30 U.S. primary care physicians to find out how they use copay coupons. Just over half of the physicians said that they use coupons between 10% and 60% of the time when they are aware that copay assistance is available. Only 10% responded that they never use coupons, while another 10% said that they always use coupons when available.
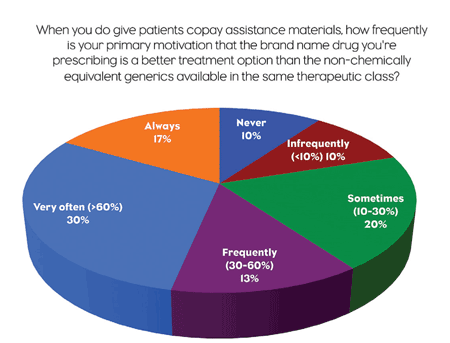
When it comes to the issue of brand-name vs. generic, 52% of physicians who do use coupons said their primary motivation is usually that the brand-name drug provides a better treatment option than the non-chemically equivalent. Only 11% claimed that that is “infrequently (up to 10% of the time)” their motivation.
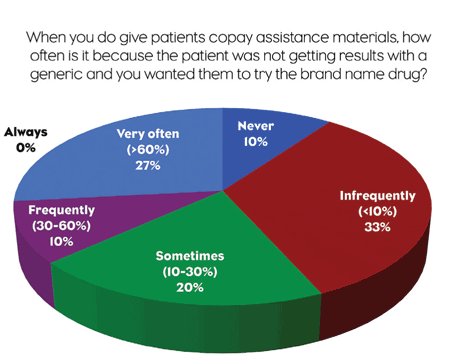
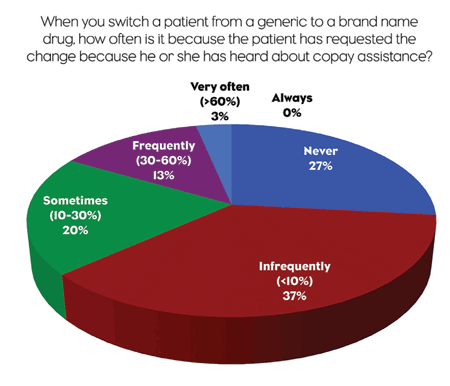
It was also very rare for these physicians to be approached by their patients about switching from a generic to a brand-name because they heard about a coupon. Only 3% responded that it happens frequently, while 63% said it occurs “never” or “infrequently.” Generally, physicians don’t try a generic first before deciding to use a copay coupon to switch to a brand. Of the physicians who do use coupons, 59% say they prescribe generics first less than 30% of the time, and 41% do it between 30% and 60% of the time.
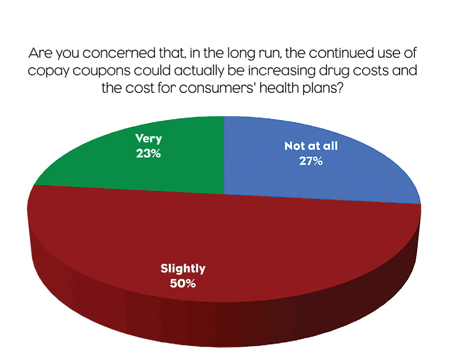
What about copay coupons’ long-term effects on healthcare costs? Half of the respondents are only slightly concerned that continued use of copay coupons could actually increase drug costs and the cost for consumers’ health plans. Meanwhile, 23% of the physicians are highly concerned about long-range impacts, while 27% are not the least bit concerned.
A Zitter Group pilot study of 43 physicians found that doctors overwhelmingly agreed copay offset programs improve patient trial or uptake as well as overall adherence. A substantial portion of the sample said they would switch prescriptions if there are two drugs that are clinically equivalent and one offers a copay discount and the other one doesn’t. About half the doctors said they had been asked about switching drugs by patients who had heard about a coupon program.
READ MORE ABOUT IT
See an expanded analysis of the Copay Issue with pharma’s take and physicians’ opinions online at bit.ly/IJIMv4.