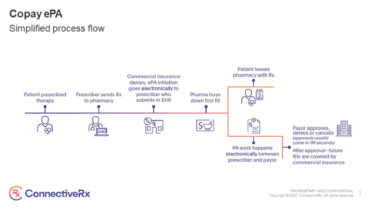
PM360’s Innovations Issue was established six years ago to serve as a comprehensive guide of the year’s most innovative Companies, Divisions, Startups, Products, Services, and Strategies. PM360 received more than 100 total submissions this year and picked the best in each category.
Here are our selections for the most innovative startups of 2017.
Alzeca Biosciences
Carlo Medici, CEO
carlo@alzeca.com
 Accurately diagnosing Alzheimer’s disease has been a significant challenge. The clinical diagnosis is highly inaccurate, and the definitive diagnosis had only been obtainable on brain autopsy after a patient’s death. Recently, research has highlighted the capability of PET scanning to detect brian amyloid plaques—a hallmark of the disease—and to more accurately pinpoint the likely onset of Alzheimer’s years before cognitive decline is detected. Unfortunately, PET scanning has its own serious limitations: It exposes those scanned to high doses of radiation, it is very costly, and very few healthcare centers have access to this technology.
Accurately diagnosing Alzheimer’s disease has been a significant challenge. The clinical diagnosis is highly inaccurate, and the definitive diagnosis had only been obtainable on brain autopsy after a patient’s death. Recently, research has highlighted the capability of PET scanning to detect brian amyloid plaques—a hallmark of the disease—and to more accurately pinpoint the likely onset of Alzheimer’s years before cognitive decline is detected. Unfortunately, PET scanning has its own serious limitations: It exposes those scanned to high doses of radiation, it is very costly, and very few healthcare centers have access to this technology.
Alzeca Biosciences is a startup bio-diagnostics company focused on developing novel, targeted MRI-based contrast agents for the early diagnosis of Alzheimer’s disease and other neurodegenerative disorders. The company’s technology platform is based on liposomal nanoparticles that carry a targeted ligand and an MRI contrast agent or other “payload.” The nanoparticles bind precisely to their target—such as beta-amyloid or another targeted protein associated with neurodegenerative disease.
With Alzeca’s technology, the deposition of such proteins in the brain can then be imaged using MRI—an approach that has significant advantages over PET, including no harmful radiation, far lower costs, far broader availability worldwide, and higher anatomic resolution. Importantly, because the platform involves no radiation, more frequent longitudinal imaging is possible, enabling the monitoring of disease progression over time—particularly critical in clinical assessment of new therapies. In addition, Alzeca’s MRI-based diagnostic agent would make widespread screening for early disease feasible.
Alzeca also plans to expand its MRI platform to include targeted imaging of tau and alpha-synuclein proteins, extending its focus beyond Alzheimer’s disease to Parkinson’s and other causes of dementia. The company recently received $11 million in Series A funding to develop its ADx nanoparticle, the first MRI contrast agent targeting amyloid plaques, through Phase 1 human clinical trials.
BioLum Sciences
Edward Allegra, CEO
ecallegra@biolumsciences.com

BioLum Sciences was founded in 2015 after its co-founders realized the potential of their university research to revolutionize the healthcare industry. So, they set out to commercialize the technology and have raised more than $1,500,000 through grants, prize, and venture investment.
The BioSense AMD (Airway Monitoring Device) will enable rapid, inexpensive, and quantitative determination of H2O2 (hydrogen peroxide) in exhaled breath, providing a valuable solution to measure airway inflammation attractive to a wide customer base ranging from biomedical research entities, to specialist doctors and primary care clinicians, and to the patients themselves. The current invention has two key innovations: 1) The composition of an optimized chemical system where the reaction can be made sensitive to a wide range of analytes through the use of a carefully designed system. 2) Methods to image, detect, and quantify the chemical emission through the use of low-cost photon detection instrumentation, and the use of the computational capacity that is an integral component of modern smartphones.
With this technology, asthma patients will have the ability to identify the presence and severity of their symptoms as a means of better understanding and improving their health, which could allow for greater treatment adherence and improved outcomes. Physicians will also have the ability to identify treatment progress with the vast amounts of personalized data generated through the device.
BioLum Sciences has performed more than four different research studies, which compare its product to the current gold standard of breath analysis—and the results have been great. One of the studies was published in a national scientific journal last year, and the company is currently preparing for a clinical trial next year to further validate the product.
CloudMine
Chris Corbet, Vice President, Business Development
ccorbet@cloudmineinc.com
Steve Wray, CEO
swray@cloudmineinc.com
 We live in a hyper-connected society. Yet, despite enormous investment in a diverse range of technology advances, healthcare remains in a largely disconnected state. CloudMine’s Connected Health Cloud gives healthcare and life sciences organizations the secure tunnels to connect and orchestrate data across critical systems that were once siloed. The platform supports agility, connectivity, and innovation for healthcare and life sciences in unprecedented ways. The Connected Health Cloud provides a secure way for the healthcare ecosystem to move and analyze sensitive protected health information (PHI) data while protecting it from the risk of intrusion.
We live in a hyper-connected society. Yet, despite enormous investment in a diverse range of technology advances, healthcare remains in a largely disconnected state. CloudMine’s Connected Health Cloud gives healthcare and life sciences organizations the secure tunnels to connect and orchestrate data across critical systems that were once siloed. The platform supports agility, connectivity, and innovation for healthcare and life sciences in unprecedented ways. The Connected Health Cloud provides a secure way for the healthcare ecosystem to move and analyze sensitive protected health information (PHI) data while protecting it from the risk of intrusion.
While pivoting exclusively into healthcare just 18 months ago, CloudMine is a pharma-proven, fully compliant Connected Health Cloud for clinical trials and integrated disease management. For example:
- CloudMine has enabled the use of mobile devices, wearables, and the integration of IoT to support and facilitate the management of remote clinical trials.
- CloudMine’s Interoperability Engine provides integrated support for a continuum of technology tools to enable patients to navigate their healthcare system journey, providing improved access to PHI, services, and support.
- The company created a foundation for a global innovation ecosystem for a leading digital healthcare organization, generating new patient and provider tools and content related to cardiovascular disease treatment and prevention.
- Partnering with a global-leading technology and content provider, CloudMine has produced a secure cloud-based environment to support a multi-location, patient-driven disease management platform.
In the last year, CloudMine has formed an array of technology partnerships that extend the company’s capabilities. CloudMine’s integrated partnerships ultimately give customers a greater level of agility. Connected Health Cloud users can build a technology framework that’s aligned with their specific needs—including solutions for the interoperability of EHR, IoT, and wearable device data.
Data Cubed
Paul Donnelly, Director – Business Development & Strategic Alliances
pauld@datacubed.com
 Data Cubed (D3) offers the next-generation of mHealth data capture, unlocking the power of clinical trials through real-world evidence. Their cross-platform app and unique hardware solutions allow them to gather a customizable selection of mobile phenomic (social, behavioral, and environmental) data points from instruments—integrating various portable and in-home technologies, customizing patient reported outcomes (ePROs), and implementing eDiaries. In the end, D3’s low-cost solutions yield more actionable data, better insights, and smarter decisions.
Data Cubed (D3) offers the next-generation of mHealth data capture, unlocking the power of clinical trials through real-world evidence. Their cross-platform app and unique hardware solutions allow them to gather a customizable selection of mobile phenomic (social, behavioral, and environmental) data points from instruments—integrating various portable and in-home technologies, customizing patient reported outcomes (ePROs), and implementing eDiaries. In the end, D3’s low-cost solutions yield more actionable data, better insights, and smarter decisions.
To create this new platform, D3 first identified four problems involving big data about humans. 1) It is not representative; 2) It’s not longitudinal; 3) It’s not deep across a broad classification of data; and 4) The only solution has generally been to stitch together fragmented data sets to try and derive more meaning. This results in biased or “frankensteined” data.
D3’s platform provides primary source data. The suite of software and hardware tools captures an unprecedented amount of phenotype data, the real-world evidence that is generating interest from every clinical and healthcare innovation group. This includes a smartphone app that pairs to a database and is licensed as a turn-key solution offering to businesses as a Platform as a Service (PaaS) model.
D3 provides research organizations the ability to deploy the app, configured to the specifics of the study, onto existing or new smartphones on either iOS or Android. Researchers receive secure daily electronic data captured from consented study participants. The system can be deployed for a new study within a day and configured to include any subset of data collection desired within the modules. Two pilots are currently underway: One with a large university (since April 2017) and one with a commercial clinical research organization (since September 2017).
EightSpokes
Andy Mehrotra, CEO
andy.mehrotra@eightspokes.com
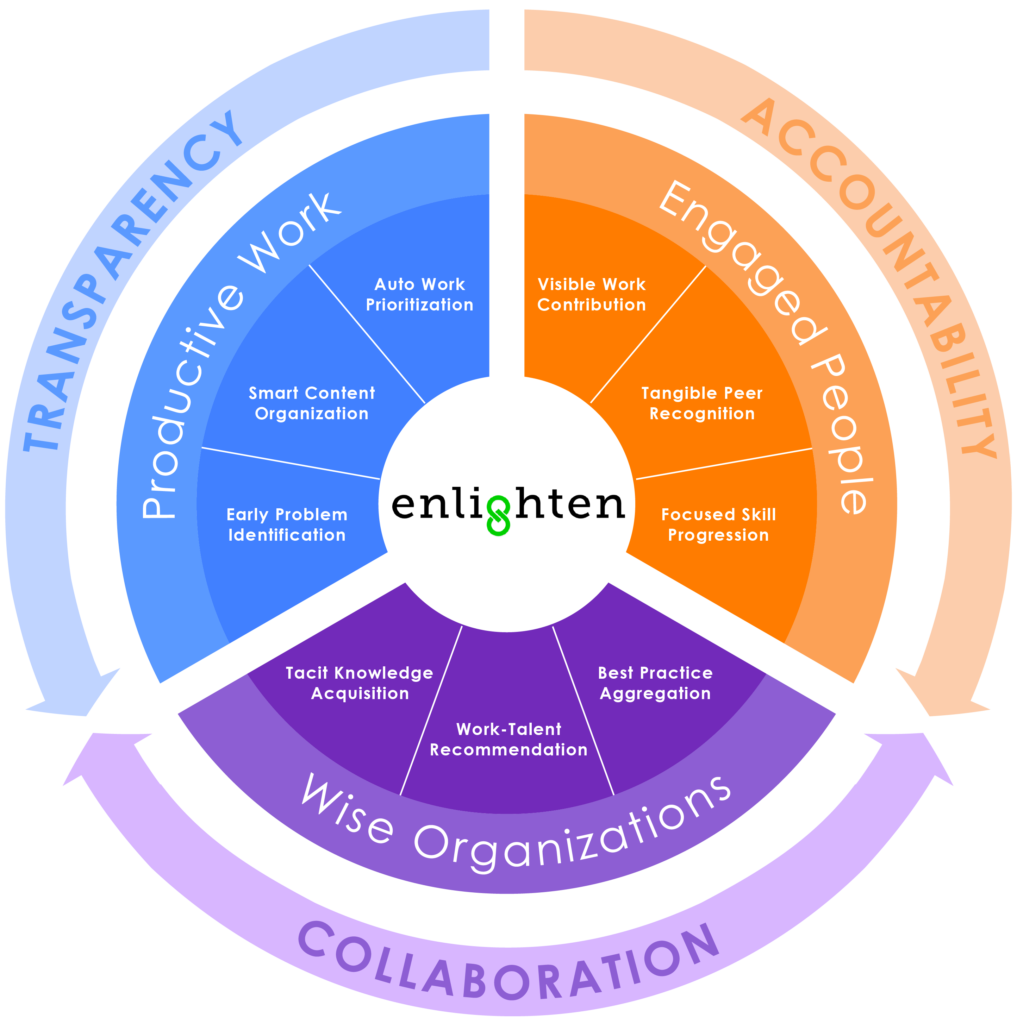 In late 2012, Andy Mehrotra, a former commercial excellence leader at Abbott, founded EightSpokes. The company’s vision: To redefine project management and create a new type of project management software designed to enable the highly complex and high-stakes projects in life sciences. The company has established a new category of software called Enterprise Project Collaboration (EPC) and formally launched its cloud-based Enlighten solution market-wide this year.
In late 2012, Andy Mehrotra, a former commercial excellence leader at Abbott, founded EightSpokes. The company’s vision: To redefine project management and create a new type of project management software designed to enable the highly complex and high-stakes projects in life sciences. The company has established a new category of software called Enterprise Project Collaboration (EPC) and formally launched its cloud-based Enlighten solution market-wide this year.
Enlighten allows companies to more efficiently manage multifaceted, multi-year projects such as new product launches, by connecting people, information, and workflows globally in the cloud. Just as important, it ensures companies retain tacit knowledge so teams can learn from projects and people that came before them, and inspires project leaders to focus on driving business outcomes—not just timelines.
Enlighten brings together all internal and external stakeholders onto a single platform for streamlined communication, coordination, and collaboration. Early adopters, such as Edge Therapeutics, have reported time savings of at least four hours per person a week.
“Enlighten is not your father’s project management software, rather it is work management software that provides an automated way to sequence daily priorities for all involved and interact with various team members in a focused way based on relevance and importance,” said Justin Zamirowski, Edge Therapeutics’ Senior Director of Operational Excellence. “It’s a genuine game changer.”
Enlighten is just starting to experience substantial growth, with nearly two dozen life sciences customers, including some of the largest global pharmaceutical companies. The company also has a dozen major consulting and implementation partners and, in the last 12 months, users from 32 countries have leveraged Enlighten.
HapYak Interactive Video
Lisa Clark, VP Sales & Marketing
sales@hapyak.com

 Through its work with many of the world’s top pharma and medical device companies, HapYak Interactive Video provides a SaaS platform that includes an out-of-the-box solution for achieving Important Safety Information (ISI) compliance in video. Previously, pharma marketing professionals were required to handle ISIs in video via custom development, costly code changes, and links out to safety information. When multiple versions were needed for different geographies, the approach did not scale. Changes were required time and again to meet the needs of each market, geography, and indication.
Through its work with many of the world’s top pharma and medical device companies, HapYak Interactive Video provides a SaaS platform that includes an out-of-the-box solution for achieving Important Safety Information (ISI) compliance in video. Previously, pharma marketing professionals were required to handle ISIs in video via custom development, costly code changes, and links out to safety information. When multiple versions were needed for different geographies, the approach did not scale. Changes were required time and again to meet the needs of each market, geography, and indication.
HapYak works with any video player, content delivery network, or video management system to add interactivity—such as chapters, branching, links, and response mechanisms—to any marketing video. This creates highly personalized and relevant video experiences proven to 10X increase viewer engagement. Easy-to-use tools let pharma marketers instantly add or modify annotations delivered from the cloud as an overlay, eliminating the need to re-cut videos to update ISI and other information.
In early 2017, HapYak extended its solution to achieve ISI compliance by requiring the viewer to watch at least 10 seconds of ISI information before a gate is released and they can navigate to other portions of the video. This enables HCPs, consumers, and others to enjoy the modern video viewing experience they have come to expect while ensuring the content complies with the presentation of ISI information in a clear, concise way, among other benefits. In some cases, chapter links may be applied and managed off-video so as not be to be intrusive to the viewer. The experience works as effectively on mobile as it does in desktop environments.
HealNow
Halston Prox, Co-founder & CEO
Halston@HealNow.io
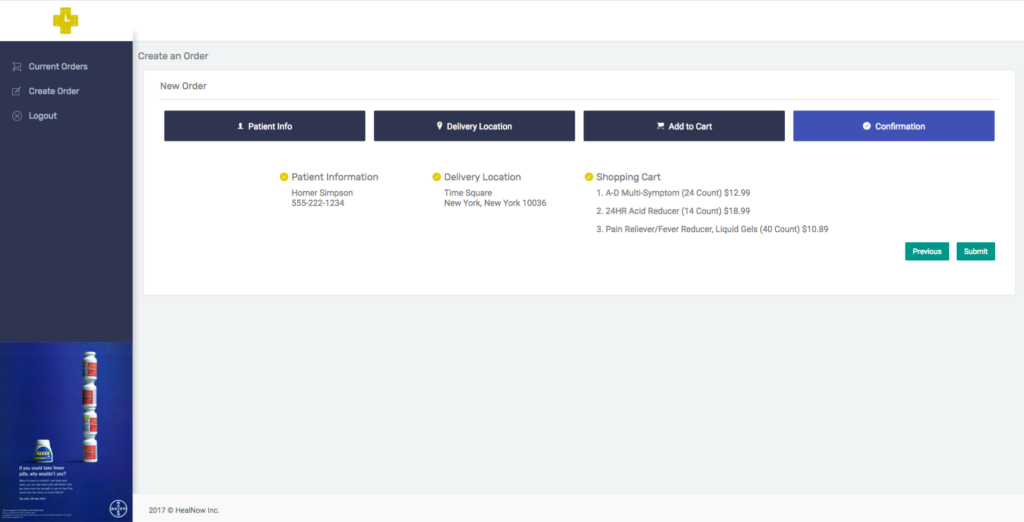
 HealNow, founded in late 2016, is a platform that enables healthcare professionals and pharmacies to offer one-hour, on-demand delivery to their patients. The company gives clinicians access to view pharmacies’ inventory in real time and order the appropriate products for their patients—along with the capability to track to see if their patients are receiving the medications/medical products they need.
HealNow, founded in late 2016, is a platform that enables healthcare professionals and pharmacies to offer one-hour, on-demand delivery to their patients. The company gives clinicians access to view pharmacies’ inventory in real time and order the appropriate products for their patients—along with the capability to track to see if their patients are receiving the medications/medical products they need.
Today, once a patient is discharged, healthcare organizations never know if their patients are receiving the products they need. With HealNow, all of that data is given back to the organization, which could lower healthcare organizations’ readmission penalties with CMS and increase prescription revenue to their pharmacy or any affiliated pharmacy. And this is all done in a HIPAA-compliant infrastructure.
The company also gives pharma marketers the opportunity to reach clinicians at the exact moment at which they’re deciding to order products for their patients. The ability to see data and trends that exist through these orders presents a huge opportunity that’s valuable to both clinicians and marketers. By advertising directly to clinicians on the HealNow platform, marketers will be able to position their brand in front of the right healthcare professional at the right time.
HealNow is focused on closing the gap between clinicians, pharmacies, and their patients, using one software. The company has already partnered with several healthcare professionals, telemedicine companies, and healthcare organizations in New York City and Austin to help bolster its offerings. So far, customers have reported being pleased with the ease of ordering products for their patients, as well as having metrics around how compliant their patient population is.
IDbyDNA
Jeff Field, Chief Commercial Officer
jfield@idbydna.com
 IDbyDNA was founded in 2014 to respond to a critical medical need: For many patients, standard lab tests never reveal the source of an infection. The founders believed that a hypothesis-free approach, built on advances in metagenomics, could provide more answers and help physicians better select treatment. The company raised $9 million in a Series A financing, led by ARTIS Ventures.
IDbyDNA was founded in 2014 to respond to a critical medical need: For many patients, standard lab tests never reveal the source of an infection. The founders believed that a hypothesis-free approach, built on advances in metagenomics, could provide more answers and help physicians better select treatment. The company raised $9 million in a Series A financing, led by ARTIS Ventures.
The unpleasant secret of diagnostic testing is just how many cases never generate results. In pneumonia testing alone, for instance, up to 60% of patients tested never get conclusive results about the cause of their infection. Was it bacterial? Viral? Fungal? The answer matters a lot for prescribing the right treatment, but for a majority of cases diagnostic assays never deliver clinically useful answers. More than 100,000 people in the U.S. die from pneumonia each year—getting better medical information quickly is essential to reducing that number.
IDbyDNA’s method comes from metagenomics, a research technique that identifies individual members of a microbial community by sequencing the population as a whole and then matching DNA and RNA results to sequences of known microbes. By applying this concept to clinical samples, lab professionals can use IDbyDNA tests to probe all organisms in a patient’s sample and quickly home in on a likely causative agent. Whether that agent is rare or common, bacterial or viral or fungal, doesn’t make a difference—they can all be found with one standard workflow.
In September 2017, IDbyDNA and ARUP Laboratories launched Explify™ Respiratory, a clinical metagenomics test for respiratory infections jointly developed and validated by the organizations. It readily identifies more than 200 common and rare causes of pneumonia and is available to ARUP’s 3,000-plus clients across the country.
IKONA
Tim Fitzpatrick, CEO
solutions@ikona.io
 IKONA, founded in March 2017, is using immersive technologies such as augmented reality (AR), virtual reality (VR), and 360° video to improve patients’ experiences and outcomes. Three years ago, the company’s co-founders conducted a randomized controlled trial that proved VR’s efficacy in reducing stress and improving patient satisfaction in the pre- and perioperative environments, respectively.
IKONA, founded in March 2017, is using immersive technologies such as augmented reality (AR), virtual reality (VR), and 360° video to improve patients’ experiences and outcomes. Three years ago, the company’s co-founders conducted a randomized controlled trial that proved VR’s efficacy in reducing stress and improving patient satisfaction in the pre- and perioperative environments, respectively.
Since then, the company built a platform to help healthcare providers create immersive solutions for educating patients and training staff. IKONA provides a vertically integrated turnkey solution for healthcare content creation.
In 2017 alone, IKONA and its collaborators have: 1) begun building a library of immersive content; 2) built a VR mobile app to manage that content library; 3) built an AR demo designed to help with treatment adherence for any patients taking prescription and OTC medications; and 4) begun exploring a store-and-forward/asynchronous VR solution for telemedicine.
The result of IKONA’s work is improving patient satisfaction and quality of care. This not only means happier patients and better-trained nurses, physicians, and staff, it also means healthcare providers can save money. It means cutting down on spending, saving providers time, improving care transitions, lowering readmission rates, lowering infection rates, and providing better care. With IKONA’s machine learning tools—such as viewer analytics and heatmapping—not only are partners better equipped to understand the needs of their patients, but patients now have more control over their own health.
Memory Labs
Aakash Adesara, Founder
contact@memory-labs.com
 Memory Labs’ mission began in February 2017 at a UC Davis hackathon where they built a voice-based memory and health aid. Since several members of their team had grandparents who went through neurodegenerative disorders, they wanted to build something that could change the way families could care for loved ones. After winning the hackathon, they thought about actually making this product a reality.
Memory Labs’ mission began in February 2017 at a UC Davis hackathon where they built a voice-based memory and health aid. Since several members of their team had grandparents who went through neurodegenerative disorders, they wanted to build something that could change the way families could care for loved ones. After winning the hackathon, they thought about actually making this product a reality.
The company is now preparing to launch Memory, a voice-based reminder assistant for the Alzheimer’s and dementia community. Memory allows caregivers to remind their patients and family members about events and pills—from anywhere in the world. The technology’s features empower seniors and provide assurance to their caregivers through two-way interaction and active dialogue.
The Memory system does the following:
- Receives pill and appointment reminders from caregiver input on an online web portal.
- Plays auditory and visual reminders on the Memory device, which seniors interact with.
- Informs caregivers when a task has been completed through SMS notifications.
The technology can also be personalized by recording and playing reminders in a loved-one’s voice as well as displaying pictures of pills and their location when it is time to take them, which can help increase patient compliance.
Caregivers also don’t need to be physically present in order to set or edit reminders—they can access their portal online to change the device’s information. And the device also supports active dialogue, meaning users can interact with the device conversationally. They can prompt Memory with questions such as “When is my next pill?” or “Can you play a song?” This allows for patient autonomy and a much better experience for elderly users.
NextWorks
George DeTorres, Executive Vice President, Business Development
gdetorres@nextworks.com
 NextWorks, LLC has created an entirely new solution for placing healthcare content with key influencers. NextWorks’ new Native Influencer Network places, monitors, and updates interactive brand experiences in contextually relevant blog posts written by key influencers. It’s powered by the company’s Content Capsule technology, which has been approved by every legal and regulatory group that has reviewed it, including 18 of the top 20 pharma companies.
NextWorks, LLC has created an entirely new solution for placing healthcare content with key influencers. NextWorks’ new Native Influencer Network places, monitors, and updates interactive brand experiences in contextually relevant blog posts written by key influencers. It’s powered by the company’s Content Capsule technology, which has been approved by every legal and regulatory group that has reviewed it, including 18 of the top 20 pharma companies.
NextWorks’ Content Capsule technology natively places interactive brand experiences (portable microsites) in client-approved editorial copy written by key influencers. The Capsule technology creates self-contained, visually interactive branded and unbranded content experiences featuring product, physician, and consumer testimonial videos, images, documents, important safety information, and calls to action. Together the content forms a valuable information package that’s delivered from a trusted source at the very moment a consumer is seeking related information. Every interaction with each piece of content on every individual blog post is measured in a client-facing dashboard. Clients can pre-determine the number of views and buy on a CPM or set fee basis.
The Content Capsule technology provides healthcare companies with complete control over content in distribution. The units contain all required fair balance information and, if changes are necessary, all content can be simultaneously updated across all blog posts and embedded locations.
In addition to being an innovative influencer marketing solution, one Content Capsule can also function as a microsite, rich media and native ad unit, and an embeddable content marketing solution that can be placed on any website where a target audience seeks information related to a brand’s message.
Prognos
Joel Keefe, SVP Life Science Sales
jkeefe@prognos.ai
 Prognos specializes in applying AI and advanced analytics to clinical diagnostics information. Since its inception six years ago, Prognos has built one of the largest lab connectivity networks in the U.S.—its key source of raw data—processed and analyzed more than 13 billion medical records for 175 million patients, and developed more than 500 proprietary machine learning-enabled algorithms.
Prognos specializes in applying AI and advanced analytics to clinical diagnostics information. Since its inception six years ago, Prognos has built one of the largest lab connectivity networks in the U.S.—its key source of raw data—processed and analyzed more than 13 billion medical records for 175 million patients, and developed more than 500 proprietary machine learning-enabled algorithms.
In 2017, Prognos launched as a new company guided by the vision to eradicate disease in partnership with life sciences and clinical diagnostics organizations as well as payers. Specifically, actionable insights derived by Prognos from lab data allow the industry to:
- Identify physicians with relevant new patients before a treatment decision is made.
- Reach out to physicians when a first-line therapy fails and the next-line therapy is evaluated.
When Prognos applies AI to lab results, it can predict with a high degree of accuracy which patient segments will respond best to a specific therapy three, six, or even nine months in advance. In one just completed project, Prognos was able to achieve a five-time increase in the probability of finding patients who will develop a certain condition that will need a particular treatment as well as predict the expected number of these patients.
That allows life sciences organizations to zoom in on the large population and stratify it by urgency, or likelihood, of requiring a therapy within a certain period of time. This enables them to guide physicians towards appropriate, optimal use either directly or through various health plan initiatives. This benefits payers, physicians, and most importantly, patients. The life sciences industry also benefits from more efficiently allocating their sales and marketing resources, better understanding the disease pattern and patient lifecycle, and identifying new growth opportunities.
Sema4
Glenn Farrell, Chief Marketing Officer
glenn.farrell@sema4genomics.com
 Sema4 was launched this year as a venture of the Mount Sinai Health System in New York. Led by Eric Schadt, the company began operations with more than 300 employees and a thriving genomic testing business spun out of Mount Sinai’s medical school and genetics lab. Now, Sema4 aims to engage consumers directly to amass enough genomic, clinical, lifestyle, and other data to drive new biological discoveries and genotype-phenotype associations. The startup is a big data play with an emphasis on predictive modeling. Already, Sema4 scientists have published the first predictive model of inflammatory bowel disease and partnered with a biopharmaceutical company for interpretation of rare diseases fueled by data science.
Sema4 was launched this year as a venture of the Mount Sinai Health System in New York. Led by Eric Schadt, the company began operations with more than 300 employees and a thriving genomic testing business spun out of Mount Sinai’s medical school and genetics lab. Now, Sema4 aims to engage consumers directly to amass enough genomic, clinical, lifestyle, and other data to drive new biological discoveries and genotype-phenotype associations. The startup is a big data play with an emphasis on predictive modeling. Already, Sema4 scientists have published the first predictive model of inflammatory bowel disease and partnered with a biopharmaceutical company for interpretation of rare diseases fueled by data science.
Recently, the company released its first consumer-ordered genomic test, Sema4 CarrierCheck. The test is targeted at young women who are interested in learning about how their DNA might affect future children. Using a saliva sample, CarrierCheck screens for 67 hereditary conditions such as cystic fibrosis, sickle cell disease, and polycystic kidney disease. CarrierCheck is available through the online marketplace at Helix, a personal genomics company. Genetic counseling is provided to help customers understand their results. Sema4 also offers expanded carrier testing that can be ordered by physicians anywhere in the U.S., and plans to launch new tests for prenatal and newborn screening.
By drawing on advances in genome sequencing, machine learning, and data interpretation, the Sema4 team sees a remarkable opportunity to improve the diagnosis, treatment, and prevention of disease. Core to Sema4’s mission is the idea that patients should be treated as partners, and that consumers who are engaged in their own healthcare will have significantly improved outcomes. The company plans to share data to help accelerate discoveries and maximize the usefulness of each patient’s contribution.
Shasqi, Inc.
Jose M. Mejia Oneto, Founder & CEO
science@shasqi.com
 Shasqi is a San Francisco-based biopharmaceutical startup company developing a novel drug delivery platform that targets drugs to specific areas of the body, enabling greater efficacy and fewer side effects. The technology, called local drug activation, uses activating agents in an injected polymer gel to concentrate and activate prodrug versions of a drug, such as a chemotherapeutic, directly at a desired location and focusing the drug’s action to that specific site.
Shasqi is a San Francisco-based biopharmaceutical startup company developing a novel drug delivery platform that targets drugs to specific areas of the body, enabling greater efficacy and fewer side effects. The technology, called local drug activation, uses activating agents in an injected polymer gel to concentrate and activate prodrug versions of a drug, such as a chemotherapeutic, directly at a desired location and focusing the drug’s action to that specific site.
In published research, Shasqi scientists have shown that the local drug activation technology can deliver the chemotherapeutic drug doxorubicin directly to cancerous sarcoma cells in mice, eliminating the tumors and the toxic side effects associated with the chemotherapy. While both traditionally delivered doxorubicin and Shasqi’s approach had an initial anti-cancer effect on the sarcoma tumors, only the locally activated drug prevented the cancer from returning.
In addition to the greater efficacy and lack of tumor recurrence, mice treated using the local activation strategy had fewer side effects, which can limit the use of combinations of cancer drugs. The researchers plan to investigate whether shorter courses of therapy using higher doses of the chemotherapeutic can be even more effective, and to expand this approach to other drugs and types of tumors.
Shasqi’s targeting method is independent of molecular biomarkers and allows drugs to be activated at a specific location for multiple weeks after the initial placement of the gel at the desired treatment site. Because the prodrug does not affect healthy cells, including immune system cells, this approach may allow the concurrent treatment with multiple cytotoxic drugs or immunotherapies.
The company is currently focused on applying its technology to chemotherapeutics and antibiotics, and is targeting its first human clinical trials for early 2019.