PM360’s Innovations Issue, established five years ago, serves as a comprehensive guide to our readers, providing a glimpse at the year’s most cutting-edge: Companies, Divisions, Startups, Products, Services, and Strategies.
Here are our picks for the most innovative startups of 2016, which include companies less than five years old pioneering new avenues in healthcare.
Circulation
Jen White, Chief Commercial Officer
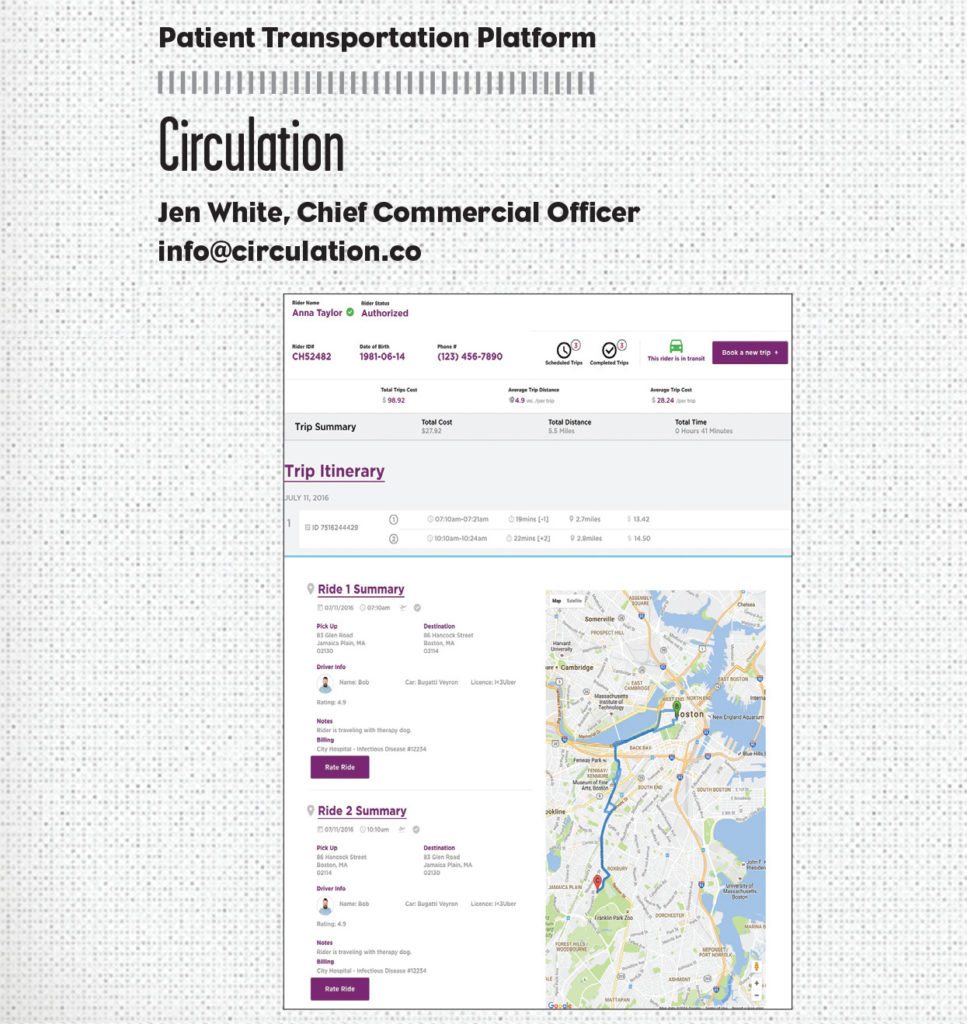
Circulation is the future of non-emergency medical transportation, created to help improve health outcomes for the estimated 3.6 million Americans (including almost one million children) who miss medical appointments each year due to transportation issues while also saving millions of dollars in wasted transportation costs.
Launched in late-September 2016 as Uber’s Preferred Healthcare Platform Partner, Circulation provides hospitals and healthcare companies with the first HIPAA-compliant, patient-centric digital transportation platform that seamlessly integrates with their systems and Uber’s API.
Hospital transportation coordinators can schedule and manage on-demand rides that are affordable and tailored around patients—all from one convenient interface. Coordinators can update patient information files with transportation scheduling information; ensure physicians, nurses, and caregivers are notified when patients arrive for appointments; and receive alerts on Circulation’s centralized multi-patient dashboard—all in real time.
While the company only just launched, Circulation is nationally available and has already announced pilots at Boston Children’s Hospital, Mercy Health System’s three acute-care hospitals and all-inclusive care program for the elderly in Pennsylvania, and Nemours Children’s Health System in Wilmington, Delaware. Circulation is also exploring expansion into clinical trials and other areas, with service rolling out across additional states soon.
Circulation was founded by healthcare experts: John Brownstein, PhD, a healthcare advisor to Uber, Professor at Harvard Medical School, and Research Faculty at Boston Children’s Hospital; Robin Heffernan, PhD, CEO of Epidemico, one of the country’s foremost population health informatics companies, and VP of Booz Allen Hamilton; Jared Hawkins, PhD, MMSc, Faculty at Boston Children’s Hospital and Director of its Informatics Innovation Program; and Leerom Segal, CEO and Co-founder of Klick Health.
Crescent Innovations, Inc.
Al Prescott, President
alprescott@crescentinnovations.com
Virtually everyone reading these pages has, or will eventually have, osteoarthritis (OA). It’s just that simple. Thirty million Americans suffer from OA—and hundreds of millions more globally. If you are lucky, over-the-counter drugs will help to manage pain and inflammation until undergoing a join replacement, but for most people with OA between the ages of 40 and 60, that is not enough.
This is the space in which Crescent Innovations, Inc. is making its first major innovative contribution. The company has developed a new and revolutionary treatment for mid-stage OA that uses none of the traditional materials currently on the market. Its technology consists of a hydrogel of poly-gamma-glutamic acid (gPGA), which in preclinical models has been shown to be safe and to reduce disease modulate inflammatory markers 20% to 30% more than currently used therapies—without side effects.
Crescent Innovations has several issued patents on its flagship technology, and in other areas including bone regeneration, advanced wound care, and more. The company is currently planning and fundraising for its first in-human studies and is looking for partners with a strategic interest in OA, tissue regeneration, advanced wound care, and more.
More specifically, the company is looking for partners willing to cast aside much of the “traditional” thinking that limits true innovation. Crescent Innovations, through its research and development efforts, has learned that much of what governs these fields is not settled science, but long-standing beliefs that simply do not stand up to a true scientific challenge. Crescent Innovations has from its founding, challenged long-held dogma in the field of regenerative medicine via out-of-the-box thinking and a rigorous scientific method.
Cyclica
Naheed Kurji, President and CEO
Pharma companies need a faster and cheaper way to discover and develop safer and more effective medicines.
Cyclica (www.cyclicarx.com) is a Toronto-based company that has developed, validated, and patented a big data and predictive analytics platform to streamline drug discovery and development pipelines. With this in mind, the Cyclica team relentlessly aims to digitize drug discovery pipelines by using its proprietary cloud-based platform.
Whether hypothetical, pre-clinical, clinical, or FDA-approved, Cyclica is able to offer insight and analysis into a small molecule’s effect. While competitors use “virtual screening” in which many compounds are screened against one protein, Cyclica’s bioinformatics and systems biology platform, Ligand Express, screens one compound against all known proteins. In so doing, Ligand Express provides a panoramic insight into a drug’s effect in the human body. Ligand Express can be viewed as a risk adjustment tool by helping to determine the likelihood of success for a small molecule earlier on in drug development, thereby saving time and money.
This approach expands the scope of reliable protein-drug interactions by a factor of 5x to 10x more than the conventional approaches, and therefore identifies novel associations, networks, and pathways that could not be identified by evaluating a single protein. This can be applied to “blockbuster” drugs as well as for rare/orphan diseases—opening entirely new markets for neglected diseases that were previously unexplored because of the high R&D expenditures required to commercialize new medicines.
The Ligand Express platform was recently introduced to the market after spending several years in research and development. This past year, the company completed numerous paid engagements, with academics, pharmaceutical, nutraceutical, and personal care companies, and is set to scale through its SaaS-based platform which will be launched in early 2017.
Deep 6 Analytics
Wout Brusselaers, CEO
Deep 6 Analytics helps healthcare innovators get cures to patients faster by tackling one of the biggest problems in drug development—finding qualified patients for clinical trials—and turning the process on its head using advances in artificial intelligence (AI).
By applying AI to patient medical records—a drastic departure from traditional patient identification methods—Deep 6 is helping researchers shave months or even years off the time it takes to advance their research and get life-saving cures into the hands of people who need them. The company also helps doctors identify clinical trials for their patients as alternative treatments when standard methods of care fall short, and gives patients more control over their health and treatment options by enabling them to find clinical trials they are eligible for based on their electronic health records.
By applying AI and natural language processing (NLP) to the medical information contained in electronic health records, Deep 6’s software finds a larger quantity of eligible patients for clinical trials faster than traditional methods, cutting recruitment delays significantly. Initial results point to a 22x increase in the speed of identifying qualified patients that meet extensive trial criteria. The number of patients found is also substantially higher due to Deep 6’s ability to analyze and understand enormous amounts of data and recognize patterns and relationships in the information. Its solution understands not just words, but also relationships between medical concepts. Additionally, the system can be trained to identify potential relationships from available data, which can guide areas of clinical research in the future—leading to new drug therapies and customized treatments.
Elevate Healthcare
Frank X. Powers, Founding Partner
Marketing is experiencing a peer-to-peer revolution. The simple idea behind companies such as Airbnb and Uber is to put people together directly without a middleman. What if you were able to take one of the key principles of the peer-to-peer commerce model—a streamlined yet robust interface between individuals to accomplish mutual business goals—and apply it to an agency model?
Elevate Healthcare is doing just that. Founded by two former top agency presidents, Lorna Weir and Frank X. Powers, Elevate eschews the traditional agency model and management hierarchy for a focus on providing strategic counsel through peer-to-peer interaction between clients and a team of multidisciplinary, deeply experienced, and visionary senior talent. This provides a comprehensive view of the complexities of a particular healthcare landscape, and the ability to move quickly to solve challenges that have a significant impact on clients’ businesses.
Elevate’s new peer-to-peer “operating system” increases transparency, accountability, and organizational agility. Its structure allows it to be what clients need, when they need it: Part strategic practice, part think tank, part idea incubator, part marketing agency.
In most marketing companies, people have exactly one job description, which is often imprecise, outdated, and irrelevant to their day-to-day work. At Elevate, senior people have flexible, even multiple, roles, often on different teams, allowing people a lot more freedom to utilize and maximize talents—and clients can take advantage of those skills in a way they couldn’t before in a traditional agency structure.
In this way, Elevate is aiming to disrupt the status quo of the agency business model in the name of improving the state of healthcare marketing—changing the definition of what a specialized agency is supposed to be, how it is supposed to operate, and what success looks like.
Enable Injections
Jeannie Joughin, Vice President, Corporate Development
Pharmaceutical and biotech companies are racing to develop promising new biologic treatments for cancers, blood disorders, and genetic and rare diseases. Biologics currently constitute 50% of products in pharmaceutical development. And startup Enable Injections is developing disruptive technology for delivering these drugs that completely transform the patient experience and also benefits pharmaceutical companies and the healthcare system.
Based on pain free injection technology, the small Oreo cookie-sized Enable Injector makes it easy, comfortable, and convenient for patients or caregivers to administer therapy at home—or anywhere. Patients are free to ambulate, work, or travel during treatment. During the approximately 30 to 60 seconds it takes for the Enable Injector to fill, it mixes and warms automatically, minimizing any potential patient error and the much-disliked 30-minute waiting time for refrigerated vials to reach room temperature. And whereas an infusion can take 30 to 90 minutes at a health facility, not including travel, it can now be completed in several minutes. All of this is expected to significantly boost compliance and, as a result, impact outcomes positively.
Pharmaceutical companies also realize several key benefits from the Enable platform, including increased profitability as the result of the greater patient adherence expected. The breakthrough technology also makes possible subcutaneous delivery of large dose (5mL to 50mL) and viscous drugs, a major pharmaceutical industry goal and chronic challenge—until now. For formulation teams, Enable’s delivery devices make development of stable, bioavailable, clinically relevant biologic formulations easier, faster, and less costly. New drug candidates can now achieve faster commercialization.
The device is currently available for investigational use only pending FDA clearance.
Genderis
Kris Marquis, Founder
Kris Marquis, a transgender woman, founded Genderis to provide a safe place where transgender people can find the resources and support they need in one organized service. The app provides people with a modern directory of the right doctors, therapists, and estheticians—a full suite of services and professionals who are compassionate and trans-friendly. The company also ensures enhanced certainty in locating qualified healthcare providers and other resources that transgender people need. Most importantly, Genderis allows transgender people to connect with providers who treat them with respect and dignity. While the product has yet to launch at press time, the company is planning to launch a pilot in December 2016.
In addition to serving as a major resource for the transgender community, Genderis is also interested in gaining a better understanding of the community by solving the questions of “who” and “why.” The “who” is the real transgender demographic. It is estimated that there are somewhere between 700,000 and 1.4 million transgender people in the U.S., but assessing some of the research done reveals that the data points to exponentially higher numbers. The company hopes that by providing a common resource, it can draw a more precise assessment of the true demographic and ultimately provide an opportunity to make things better once businesses and the government understand the true number.
The “why” is: “Why are we like this?” As Marquis explains, she has always asked this question and the answer from many therapists is that “you simply are,” with little or no explanation. Genderis believes that through the volumes of users, generic data patterns will arise that will provide more dimensional causation and timelines pointing to possible common indicators.
InTask, Inc.
Greg Chu, Chief Operating Officer
InTask is a market research software that delivers deep insights into physician decision-making and behavior. Introduced in early 2016, the software platform overcomes many of the limitations of current survey methodologies while enabling new solutions to some of the industry’s most difficult research problems.
The InTask platform engages physicians in a simulation built around the management of virtual patients, generating research data by recording every action taken by the physician in the course of diagnosing, treating, and managing these patients. Patients return for multiple visits in virtual time, creating rich longitudinal patient treatment data that can be used to drive insights into both treatment algorithms and drivers of physician decision-making. Flexible choice architecture allows the InTask platform to simulate and assess the impact of environmental scenarios such as the availability of new products, changes in access, and exposure to promotional messages.
From the respondent perspective, the InTask platform has proven to be more engaging and interesting than traditional questionnaires. Inspired in part by core principles of game design, the simulation mimics real-world activities in a way that makes sense to physicians and stimulates their best thinking. Pilot tests have shown that 9 of 10 physicians find the platform more interesting than traditional surveys.
InTask, Inc. was established in 2016 to commercialize this platform. Since its first commercial application, InTask has been used in a range of market research applications in both the U.S. and internationally. It is also being adapted for other applications, including sales force training, CME, and managed markets modeling. To speed the uptake of the software, InTask, Inc. partnered with SHC Universal to make the technology available to any healthcare market research agency.
InTouchMD
Ryan Alovis, CEO
InTouchMD, founded in 2012, is a single-source healthcare solutions company that has successfully bundled three offerings: Data services (patient and HCP), outreach tactics (email, tele, SMS, fax, banner, direct mail), and marketing automation. But what makes InTouchMD truly unique is its in-house development team that has successfully created the first-ever, hyper-focused healthcare marketing automation platform, Pulse.
InTouchMD’s Pulse platform, with the latest version of the platform released in 2015, allows pharmaceutical companies the ability to leverage real-time user actions to better understand how and when they should be reaching their target. The company has seen anywhere from a 15% to a 20% lift in engagement by clients that utilize the Pulse system—and it’s allowed companies to save a tremendous amount of money. The thesis is to allow pharmaceutical companies to market smarter and push for zero waste.
The challenge for InTouchMD when it first came onto the scene was the whole “no new friends” constraint. A lot of companies will only work with you if you’ve worked with other companies in the past, and it can be even more difficult when offering a new technology. But now InTouchMD counts some of the top companies in the industry as clients.
Ultimately, through its offerings, InTouchMD hopes to end the days of working with multiple vendors to accomplish multiple things. Through InTouchMD, you can buy premium data with the same company that’s handling the outreach, while utilizing its propriety marketing automation platform to increase engagement precision.
MetaMe Health
Danny Bernstein, Co-founder
IBS (irritable bowel syndrome) is a chronic condition with symptoms that include diarrhea and constipation, or both, along with bloating and abdominal pain. MetaMe Health delivers a clinically proven treatment for IBS without the use of drugs, without the need to travel to an appointment, and at less than half the cost of traditional office visits.
Current drugs are effective for only about 25% of IBS patients. In contrast, 10 randomized controlled trials (RCTs) show that medical hypnosis provides relief to 70% of IBS patients with an average reduction in symptom severity of 50%. MetaMe Health holds the worldwide exclusive license for online use of the only clinically proven, standardized medical hypnosis treatment for IBS. The company enables delivery of that treatment through its telemedicine platform, MetaMe Connect (www.metameconnect.com).
MetaMe Connect offers state-licensed therapists: A sublicense to deliver the clinically proven IBS protocol online; a HIPAA-compliant telemedicine platform for video conferencing with patients; professional video recording of the therapist delivering hypnosis sessions; secure hosting of pre-recorded videos for delivery to patients; scheduling services; billing services; and marketing services (one-minute product video: https://vimeo.com/187225549). Therapists work with MetaMe Health at their convenience, from the comfort of their home or office, and therapist compensation is competitive with a traditional office practice.
MetaMe Health is currently operating in Illinois, with plans to expand to other states within the next 12 months and nationwide within three years. MetaMe Health is currently seeking partners to explore use of its treatment as an adjuvant to pharmaceutical approaches, so that a combination therapy can be in place as the company achieves nationwide coverage.
Patient Pattern, Inc.
Steven Buslovich, Co-founder, CEO
Started in 2013 by a geriatrician and serial IT entrepreneur, Patient Pattern, Inc. was created in response to personal experiences of inefficiencies within nursing home and post-acute care facilities. Joined in 2014 by a geriatric nurse practitioner and public policy/statistician, they began to focus their attention from consumer-facing products to their current market by developing the LivePAC suite.
LivePAC addresses the clinical care needs and the organizational pain points of chronically ill, older patients residing in nursing homes and post-acute care rehabilitation units. The product identifies those who will do better in rehabilitation and those who are less likely to complete the program because of their underlying frailty. Calculating, in real time, the patient’s degree of frailty and communicating their associated vulnerability to bad outcomes to their doctors begins the process of providing patient-centered care and informed decision-making.
For example, last year LivePAC was implemented by a 300-bed nursing home that admits about 680 patients per year. The anticipated 30-day hospital readmission rate based on their current metric was 24%. Within six-months of implementation, the reduction of 30-day all-cause readmissions was substantial with readmissions decreasing from 24% in early 2015 to 11% by the second quarter of 2016—a 55% reduction.
Clinicians received actionable evidence-based and geriatric expert recommendations for addressing each poor outcome, and this led to a 25% to 72% decrease in critical quality outcome measures. With a reduction in poor outcomes, there was a parallel reduction in utilization costs. Pharmaceutical costs were decreased by $600,000, laboratory costs decreased by approximately $210,000, and supply costs decreased by $100,000 within one year.
Protenus
Robert Lord, Co-founder and CEO
The healthcare industry suffers more cyber breaches than any other field, with more than 111 million records breached in 2015 alone. By its very nature health data can be especially catastrophic because it contains information which is nearly impossible to change or reset upon a breach. Furthermore, this data contains a complete picture of a person’s life, and can be used for a terrifying array of threats—including identity fraud, medical blackmail, Medicare fraud, and prescription fraud.
Protenus (http://bit.ly/2gnaQSe), founded in 2014, delivers a new approach to the patient privacy monitoring challenge by using the latest big data techniques and Protenus-led advances in data science, machine learning, visualization, and engineering. With the Protenus platform, hospitals can analyze hundreds of threat dimensions to provide privacy and security officers a continual 360° view of all access to their EHR system.
The Protenus patient privacy analytics platform, at work in industry-leading hospitals such as Johns Hopkins, understands the difference between a cardiologist and a research nurse, a diabetes patient and a critically ill admission, and a surgical ward and an outpatient clinic. This important context allows hospitals to find user access patterns that might otherwise remain hidden, and perfectly reasonable explanations that might have otherwise taken hours of investigation to determine.
Protenus also incorporates an understanding of the complex clinical environment by replacing the manual process of sifting through false positives with a greater than 97% accuracy (compared to a present standard of roughly 17% or less accuracy, based on a recent case study). In the end, Protenus streamlines threat detection and resolution cycles from months to a matter of minutes.
QuiO
Alexander Dahmani, Co-founder & CEO
QuiO (pronounced “kwee-oh”), founded in 2014, provides smart injection devices and connected software for monitoring, engaging, and supporting patients taking injectable therapies, such as biologics and biosimilars, which are the fastest growing segment of the pharmaceutical market. However, injectable therapies can be difficult to administer at home and there are no solutions available for monitoring patient adherence to these therapies.
QuiO has created the first real-time adherence monitoring solution for injectable therapies. The Smartinjector device platform is the first fully connected drug delivery solution, enabling remote monitoring of dosing events and injection performance without any syncing or smartphone required.
The patient simply uses the device to self-inject a dose of their prescribed therapy, and the Smartinjector will record how much drug was loaded, how much was delivered, and a timestamp of the dosing event. Additionally, Smartinjector devices will immediately and passively send data through a secure long-range wireless connection. The data is used to deliver automated support, target high-touch interventions, and track real-world drug performance.
QuiO is in the final stages of development and testing for its first Smartinjector device, the Si One, which is a handheld reusable injection device for syringes. Thanks to a proprietary design and dynamic injection mechanism, the Si One is a general use injector that is compatible with all syringe-based medications.
QuiO also has reusable, disposable, and wearable Smartinjector devices under development—all of which are covered by a robust IP portfolio. Smartinjector devices are easy and reliable for all patients to use, which will improve clinical trials and disease management programs through remote monitoring of home injections.
Slope.io
Rust Felix, CEO
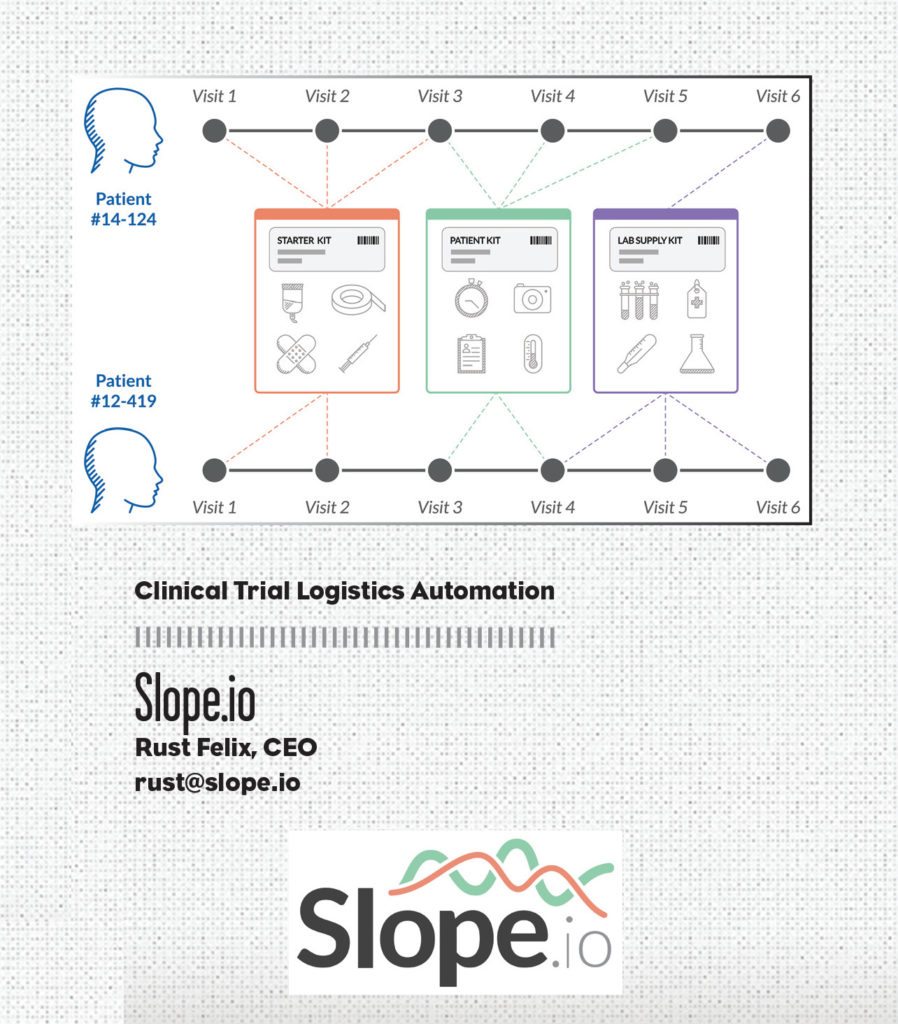
Researchers are passionate about improving people’s lives, not figuring out supply chain problems. Slope.io helps researchers by ensuring their network of patients get the exact ancillary clinical supplies their care requires on time, on schedule, and on budget. The company leverages logistics automation, predictive forecasting via EDC integration, and more than 10 years of service design expertise to save clinical trials time and money while increasing research site efficiency and supply chain visibility. Its clinical trial supply kitting service is fully managed, providing a drop-in turnkey solution that functions in the background, eliminating the complex web of communications traditionally required to keep research sites fully provisioned.
As research site professionals will tell you and trial analytics prove, happier study sites are higher patient enrollers—and tend to have better patient retention. Slope.io helps build happy, productive clinical research sites by empowering them with tailored clinical supply kits automatically delivered just in time before each patient visit. Slope.io’s forecasting algorithms leverage real-time trial data to anticipate patient supply demand, tracking every patient visit across each study site. Slope.io helps researchers focus on the science, not the logistics.
The technology behind Slope.io began more than six years ago as an eCommerce forecasting engine used for predicting product demand. In Q1 2016, clinical trial business logic was applied to the forecasting toolchain and the service was successfully delivered for a pilot client on a Phase II trial. Since its product launch in Q2, Slope.io has scaled operations and launched five more Phase I/II trials, delivering more than 2,000 supply kits to more than a hundred research sites across the U.S.
Tetra Discovery Partners
Mark E. Gurney, PhD, CEO
Tetra Discovery Partners is a clinical-stage biopharmaceutical company developing a portfolio of drugs to treat Alzheimer’s disease, depression, and traumatic brain injury. The Tetra team was the first to solve the protein structures of the regulatory domains of the phosphodiesterase type 4 (PDE4) enzymes. This enabled Tetra to design, for the first time, drugs that are PDE4 subtype-selective allosteric inhibitors.
There has been great interest in the PDE4 family as the enzymes are key modulators of cAMP signaling. In the brain, the PDE4D subtype modulates the spatial and temporal patterning of cAMP signaling important for learning and memory. After brain trauma, the PDE4B subtype exacerbates inflammation and thereby, brain injury.
Tetra has advanced the first subtype-selective, PDE4D allosteric inhibitor into human clinical trials. Animal studies indicate a superior procognitive profile with very wide safety margins. Human Phase I studies ongoing this year are seeking preliminary indication of cognitive benefit in older subjects (>60 yrs.) with the potential to treat age-associated memory impairment, mild cognitive impairment prodromal to Alzheimer’s disease, and Alzheimer’s disease. By facilitating the formation of new connections between brain neurons, the PDE4D allosteric inhibitor has the potential to modify the progression of Alzheimer’s disease.
The development of BPN14770, the Tetra memory drug, was through a cooperative research agreement with the National Institutes of Health Blueprint Neurotherapeutics Program. The joint program, which began in 2012, successfully opened an Investigational New Drug (IND) application with the FDA in 2015. Rapid chemical optimization of BPN14770 was achieved through reiterative use of structure-based drug design. BPN14770 has high oral bioavailability, linear exposure with dose in human subjects, and outstanding tolerability and safety margins.
Vivor
Melissa Thompson, Head of Strategy
Out-of-pocket healthcare costs have risen dramatically in recent years and this affordability crisis has even led to the coining of a new term in medical literature: “Financial Toxicity.” This problem is particularly acute for patients with expensive diseases such as cancer, in which patients are 2.6x more likely to experience bankruptcy—a significant risk factor for mortality.
Vivor builds software that directly addresses financial toxicity, with a particular focus on high-cost drugs. Pharma manufacturers and foundations offer generous co-pay assistance programs, yet these programs remain underutilized despite proven need. Vivor’s PayRx, launched in June 2015, is a proprietary platform that instantly matches each unique patient profile with a personalized set of financial assistance programs. The platform is accessible through PayRx Navigator (Vivor’s own web app for financial navigators) or PayRx Connector (an API-based system designed to integrate with EHRs and other enterprise software).
Vivor’s platform creates value for:
- Patients: Lower out-of-pocket costs result in increased access to care, greater adherence, and better health outcomes.
- Pharma Manufacturers: Out-of-pocket costs are the top non-clinical barrier to prescription initiation and adherence. Vivor streamlines the process of identifying and enrolling in co-pay assistance, reducing access barriers, and enabling appropriate user therapy.
- Healthcare Providers: When providers increase utilization of co-pay assistance—especially for buy-and-bill drugs—they reduce balance after insurance (BAI) that often leads to bad debt. Providers also benefit by improving the patient experience—a metric of increasing importance.
Vivor is the recipient of a prestigious $1.73 million multi-year STTR Fast-Track grant from the National Cancer Institute/National Institutes of Health to develop and test financial toxicity interventions in partnership with the Duke Cancer Institute.