PM360’s Innovations Issue, established five years ago, serves as a comprehensive guide to our readers, providing a glimpse at the year’s most cutting-edge: Companies, Divisions, Startups, Products, Services, and Strategies.
Here are our picks for the most innovative companies of 2016, which include any organization developing new ways to push the industry forward
81qd
Daniel Leszkiewicz, PhD, Senior Vice President, Health Sciences and Applied Analytics
Growth in healthcare data is occurring at an exponential rate, and biopharmaceutical and medical device companies are struggling to access, manage, and leverage insights from the information to achieve their objectives. Machine learning-based algorithms can be used to harness the power of patient-centric datasets to produce actionable outcomes. Such models are able to integrate data identifying the most influential clinicians and their networks and serve as a catalyst to help clinicians identify undiagnosed patients and shorten the time to diagnosis. 81qd, an advanced healthcare data analytics company, offers two recently introduced solutions that leverage patient-centric analytics through the integration of clinical network mapping and patient finding to address multiple business challenges facing the biopharmaceutical industry.
Its Plexus analytic solution harnesses the power of the vast amounts of information contained within healthcare encounter data. The application of machine learning techniques enables 81qd to uncover actionable insights that would otherwise be virtually impossible to find. Plexus objectively and quantitatively identifies the most influential clinicians that measurably impact the clinical behavior of other clinicians. Importantly, the solution also provides a map of the clinical networks of these influential clinicians and measures the relative strength of the connections.
Meanwhile, 81qd’s predictive analytics tool, Orion, can facilitate the identification of a yet-to-be diagnosed patient with a specific rare disease. The solution leverages machine learning and computational statistics to analyze disparate sources of data, including healthcare encounter data, laboratory data, and electronic health records, to reliably predict which patients have the highest probability of having a particular rare disease and identify the specific clinicians who are currently managing those very patients for other conditions.
ADM Diagnostics
Midori Yokoyama, PhD, Vice President, Business Development
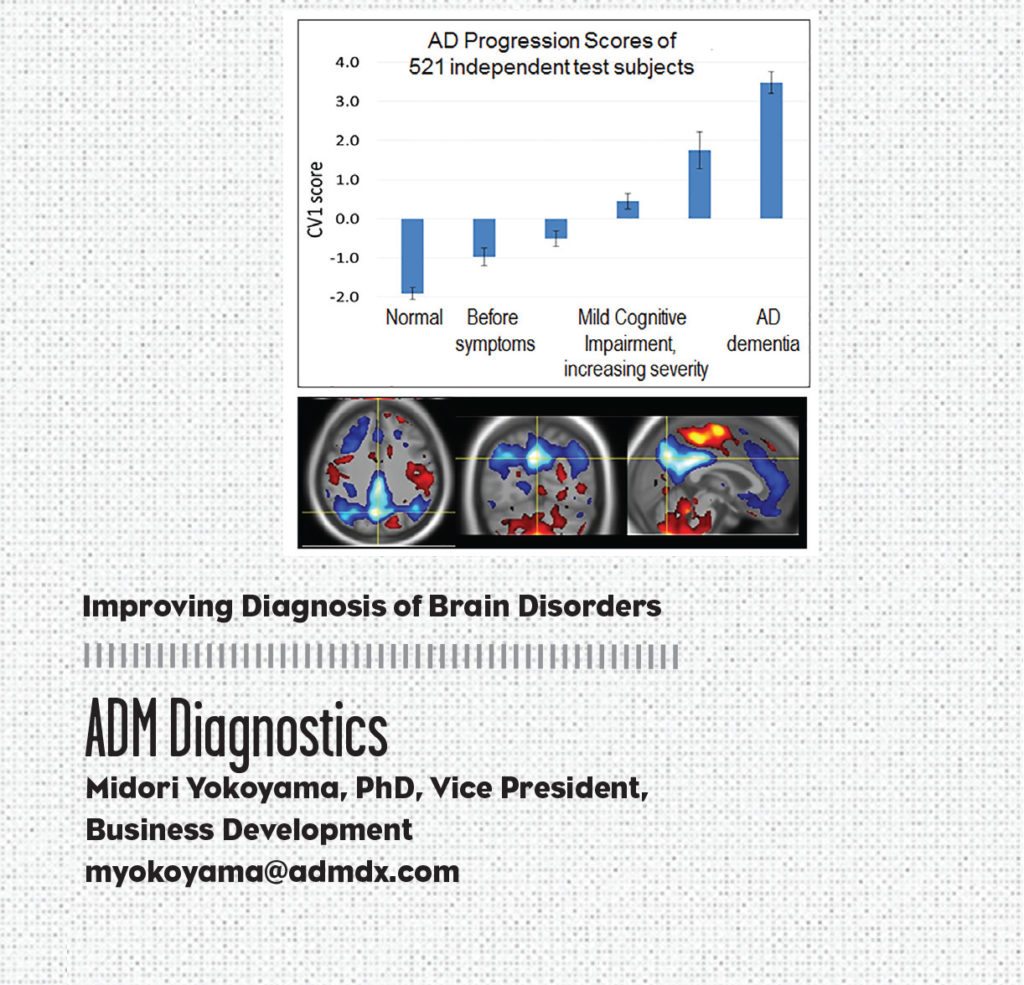
ADM Diagnostics (ADMdx), a Chicago-based company, has developed advanced brain image analysis technology to enable early and accurate diagnosis, prognosis, and detection of treatment effect for Alzheimer’s disease (AD) and other brain disorders. ADMdx’s innovations are in the development of advanced machine learning algorithms and software to identify patterns of brain activity and structure that reveal disease in its earliest stages—even in pre-symptomatic stages.
The development of effective therapeutics to treat dementias poses an immense need as AD affects more than five million people in the U.S. today and is projected to reach more than 130 million worldwide by 2050. And misdiagnosis of Alzheimer’s patients, ranging from 20% to 50% according to the Alzheimer’s Association, has impeded clinical trials as well as appropriate clinical care. But ADMdx’s technology can help address those issues thanks to its ability to identify and differentiate disease, predict rates of clinical worsening, and quantify effects on brain networks associated with treatment. This can help increase the opportunity for clinical trial success—and to support effective therapeutic use in clinical care.
ADMdx has developed imaging classifiers using Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) to differentiate dementias, identify disease stage, and provide prognostic insight. Early evaluations of the company’s dementia differentiation classifier showed that it is 97% to 99% accurate in differentiating AD from other forms of dementia.
Currently, ADMdx provides imaging studies and data analysis to Fortune 500 pharmaceutical companies and biotechnology companies that are developing new therapeutics for AD, Parkinson’s disease, and other brain disorders. ADMdx’s technology is being used to help determine patient inclusion in clinical trials, stratify patient populations for comparison of drug and placebo, and detect treatment response.
ContextMedia:Health
Philip de Guzman, Communications Lead
philip.deguzman@contextmediainc.com
ContextMedia:Health is ending 2016 on a big note with the acquisition of AccentHealth, which will make it the largest healthcare decision platform at the point of care in the U.S., with a presence in 55,000 offices. Even before the acquisition, ContextMedia:Health was having a good year expanding its reach across clinics, hospitals, and health systems by adding nearly 1% of all practices in the country each month. Currently, ContextMedia:Health is tracking to be in 70% of all practices, hospitals, and health systems by 2020.
Much of that success can be attributed to three award-winning products built within the last year: Patient WiFi (now marketed under the brand, Triggyr), the Infusion Room Tablet, and the Digital Exam Room Wallboard. Triggyr offers brands the ability to reach patients on their personal mobile device using the in-office WiFi. The Infusion Room Tablet was designed with the input of cancer support organizations to truly understand the infusion room experience in order to curate content that was encouraging, positive, and empathetic to the patients in that context. And the ContextMedia:Health Wallboard gives physicians access to dozens of 3D anatomical diagrams and videos of all the systems of the human body as well as numerous condition states in order to improve information retention and comprehension with their patients.
A recent Quality Resource and Use Report, conducted by the Centers for Medicare & Medicaid Services, found that an office integrated with ContextMedia:Health technology reported an 82% lower rate of hospital cases for urinary tract infections, a 58% lower rate for patients with diabetes, and a 33% lower rate for those with chronic obstructive pulmonary disease or asthma compared to the national average.
Greenphire
Wayne Baker, Chief Commercial Officer
Greenphire was founded in 2008 to address the burden that manual payments placed on the clinical trial process. When it comes to clinical trials, an industry-wide pain point is inefficient, inaccurate manual reporting processes and frustrating payment experiences that make it difficult to retain the best sites and patients.
Greenphire’s eClinicalGPS solution streamlines and accelerates global site payments through a centralized, web-based solution, which allows sponsors and CROs to eliminate the time-consuming, error-prone manual processes, which can slow down site operations. In addition, the automated payments means more time for sites to focus on research and the performance of the study, instead of chasing down payments.
The company has also changed how clinical trial participants are reimbursed. Rather than receiving cash (easy to lose) or checks (taking months to receive), Greenphire’s ClinCard is a reloadable debit card that is easy to use and accepted worldwide. This patented payment technology has been used by thousands of research sites to reimburse nearly 400,000 patients across more than 25 countries, automating payments of more than $200 million. Furthermore, through Greenphire’s ClinCard Travel Module, study participants are able to travel for the study without incurring out-of-pocket cost, further eliminating any hurdles that may keep the patient from participating in the study.
And in 2016, Greenphire took its ClinCard solution to the next level, expanding its usability to more countries than ever before. This expansion allows the company to reimburse global clinical trial participants via a reloadable debit card in their local currency. It also delivers global trials the financial transparency and consistency to support compliance and reporting requirements.
Healthcasts
Debra Harris, Sr. Director Marketing Strategy
In 2016, Healthcasts celebrates 15 years as a company that has built its success with a winning formula that reveals what doctors want to know and marries it to what marketers want to communicate. By listening to and gathering feedback from physicians, Healthcasts has revolutionized the way that doctors learn, and the past year has only offered more innovation from the company.
The Healthcasts experience was enhanced with new interactive products that educate physicians beyond video—which until recently was the sole sponsor opportunity on the platform. Delivered sequentially in a customized and personalized “HCP Journey” on Healthcasts—only to the target physicians that will benefit from this information—these new products, in combination with engaging video, allow physicians to access needed information from pharma without being overwhelmed with too much information. It also affords sponsors the opportunity to benefit from ongoing physician feedback on content and messaging strategy.
Products are customized to meet both pharma and physician needs and may focus on clinical data, patient identification, sales rep connection, sample access/formulary information, and more. Key capabilities were also enhanced this year such as tailoring programs to complement initiatives outside of Healthcasts (such as speaker events), targeting outreach for both target specialists and their referring physicians with customized messaging, and improving client target list/segmentation initiatives.
Healthcasts also boosted its “Physician Voices” initiative, which is focused on connecting the pharma community with insights gleaned from the company’s esteemed thought leaders and influential member physicians. In the coming months, Healthcasts plans to use Physician Voices to further improve communication between physicians and pharma.
InnoCentive
John Elliott, Business Development
Over the past 12 months, InnoCentive—the open innovation and crowdsourcing pioneer—has partnered with leading pharmaceutical companies such as Boehringer Ingelheim and AstraZeneca to help them crowdsource innovative solutions to their pressing problems. Successful examples include sourcing new therapeutic approaches for treating diabetic nephropathy and a device for the transplantation and subsequent retrieval of human islets for regulating blood glucose levels in diabetics.
All of this was made possible through InnoCentive’s unique approach that combines its Challenge Driven Innovation methodology, its global network of 375,000+ problem solvers, and its purpose-built platform. Organizations that work with InnoCentive to solve “Challenges” get more than a rapid and cost-effective way to find innovative solutions; they are connected with scientists and researchers from around the world, raising their profiles as leaders in science and innovation. InnoCentive Challenges currently operate a very high success rate, with solutions being awarded in more than 80% of cases. A study by Forrester Research found that InnoCentive Challenges brought a return on investment of 74% for clients, with a payback period of less than three months.
InnoCentive’s Solver network continues to grow in both size and diversity: In 2005, for all Challenges run, winning Solvers originated from only 11 countries. By 2010, it was 36 countries. Last year, it was 47. In just the first three months of 2016, Solvers were awarded from more countries than in the entirety of 2007. High-profile Challenges that could have a major impact on the pharma industry are also continuing to be launched, such as Elanco’s Grand Challenge Program—a series of Challenges looking for alternatives to animal antibiotics.
JUICE Pharma Worldwide
Robert Palmer, EVP, Digital Innovation Officer
JUICE Pharma Worldwide recently developed several groundbreaking projects that are turning standard interactions into highly personalized experiences. The first is in HIV-1, where the condition has become increasingly manageable and patients are developing conditions typically associated with aging. As a result, many HIV-1 patients are taking medications for diseases such as high blood pressure, diabetes, high cholesterol, and mood disorders. As this dynamic is quite new for physicians, they needed help staying on top of the potential problem of drug-drug interactions (DDI). JUICE tapped into big data on the local level to create the DDI iPad tool that enables sales reps to show physicians at the ZIP code level the percentage of patients in their area who filled prescriptions for HIV-1 treatments in addition to other prescription and non-prescription products with the potential to cause DDIs.
Inspired by the personal nature of using message- and voice-activated platforms to chat with friends, order food, schedule appointments, and research products, JUICE incorporated this Chatbot technology into the Orencia Rheumatoid Arthritis (RA) Assessment Tool. Instead of a flat experience, the patient engages with the RA Assessment Tool in a chat interface, where the “person” on the other end leads the patient through a series of questions that flow logically based on the patient’s personal responses.
The exchange covers patients’ understanding of their own diagnosis, pain level, symptoms, treatment regimen, and insurance types. In the end, patients are given a choice of how to see and receive results of their “conversations.” Patients can view a PDF within the browser window and have their results emailed to them.
Kognito
Ron Goldman, CEO
Kognito is a NYC-based company that creates role-play simulations to prepare health professionals, patients, caregivers, and others to effectively lead real-life conversations that drive measurable improvements in social, emotional, and physical health. Kognito simulations engage users in practice conversations with emotionally responsive virtual people that are coded with individual personalities and memory. In 2016, Kognito released two simulations that address common but challenging conversations between physicians and patients.
The first simulation, funded by the Robert Wood Johnson Foundation, is entitled The Primary Care Office Visit: Antibiotics, available at www.conversationsforhealth.org. The role-play simulation allows users to choose whether to play the physician or the patient. As the physician, users talk to a virtual patient and see how well they do in building trust, collaborating on a treatment plan, and addressing the patient’s request for antibiotics. As the patient, users learn about the proper use of antibiotics, how to make sure they get answers to their questions, and leave the office with a plan that works for them. At the conclusion, a personalized performance dashboard provides the user feedback on their performance in the conversation.
The second simulation was Change Talk: Childhood Obesity, available at www.kognito.com/changetalk. This simulation, developed in collaboration with The American Academy of Pediatrics (AAP) is designed to prepare pediatricians to lead real-life conversations with children and their parents about childhood obesity. Data collected by the AAP from 307 pediatricians shows that one month after completing the simulation, 88% indicated they changed how they interact with patients on this topic. One physician said, “I usually lecture patients and see little change. This was amazing and a whole new way of thinking and communicating.”
Noninvasix
Dr. Graham Randall, CEO
Founded in 2007, Noninvasix is backed by 15 years of optoacoustic research by its co-founders and more than $6.5 million in NIH research grants. The company’s goal: To reduce the incidence of neonatal mortality and morbidity by developing a patient monitor to accurately and noninvasively measure brain oxygenation in preterm and low birth weight babies.
With patented optoacoustic technology at its core, Noninvasix’s system pulses laser light into the brain to measure the amount of oxygen the baby is receiving in real time. Prompt recognition of low cerebral venous oxygenation can be used to guide therapeutic interventions and reduce adverse outcomes.
A sensitive wide-band headstrap accesses the baby’s front and back fontanelles, and pulsing frequencies of near-infrared light are sent into the brain’s Superior Sagittal Sinus vein. Hemoglobin in the blood absorbs the light at different frequencies depending on whether or not it is carrying oxygen. Absorption causes rapid thermal expansion of the hemoglobin resulting in a measurable acoustic wave.
In contrast to other purely spectroscopic techniques, such as near-infrared spectroscopy, this technology provides an absolute, rather than relative measurement, and can be targeted to specific blood vessels. Demonstrated in in vitro, animal, and clinical tests, Noninvasix’s prototype measures cerebral oxygenation (SO2) in individual brain blood vessels accurately and precisely (correlation: r2 = 0.99; bias = 2.47%; SD = ±2.3%) in comparison to invasive hemoximetry—the gold standard for those measurements.
Noninvasix recently completed a 510k pre-submission with the FDA, and agreed on a de novo 510k pathway to FDA clearance. Noninvasix’s next step is to build the manufacturing prototype, to take to clinical trials and the FDA for regulatory clearance, which is expected in 2017.
Procyrion, Inc.
Ben Hertzog, CEO
At the earliest signs of heart failure, patients are given a cocktail of drugs, each one managing a different symptom. As the disease progresses, patients often become refractory to medical therapy and are faced with rapidly declining quality of life. However, the only other options available are not suitable for early or widespread use: Left Ventricular Assist Device (LVAD) implants are risky and expensive and heart transplants are in limited supply. As a result, these options are only used as a last resort.
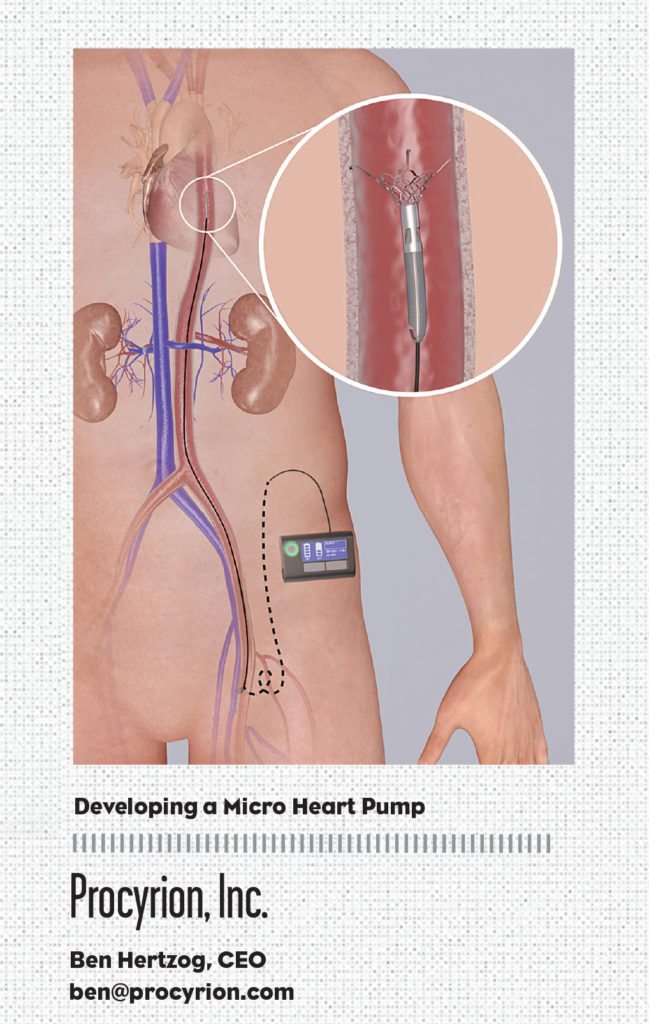
Founded in 2005, Procyrion, Inc. has identified an opportunity in the sweet spot—the time between when the drugs start to fail and a last-resort surgical intervention—to treat millions of NYHA Class III-IVa heart failure patients using a novel and minimally invasive catheter-based heart pump, Aortix. This groundbreaking interventional cardiology tool, conceived by cardiologist Dr. Reynolds M. Delgado, III, Medical Director of Mechanical Support Devices in Heart Failure at the Texas Heart Institute, was designed to reduce the workload of the failing heart, which allows the muscle a chance to rest and heal.
Positive pre-clinical test results, including a 40% reduction in the workload of the heart and increased flow to vital organs such as the kidneys, shows that the powerful micro pump could offer a new, high-quality-of-life therapeutic option for these more active patients. It also has the potential to lower the $35 billion annual spend on heart failure by cutting treatment costs and readmission rates.
Investors agree in the device’s potential as Procyrion recently completed a $10M Series B financing round. That money will fund the company beyond its first-in-human studies, which are planned for late 2016.
RenovaCare, Inc.
Dwain Schenck, Vice President, Corporate Communications
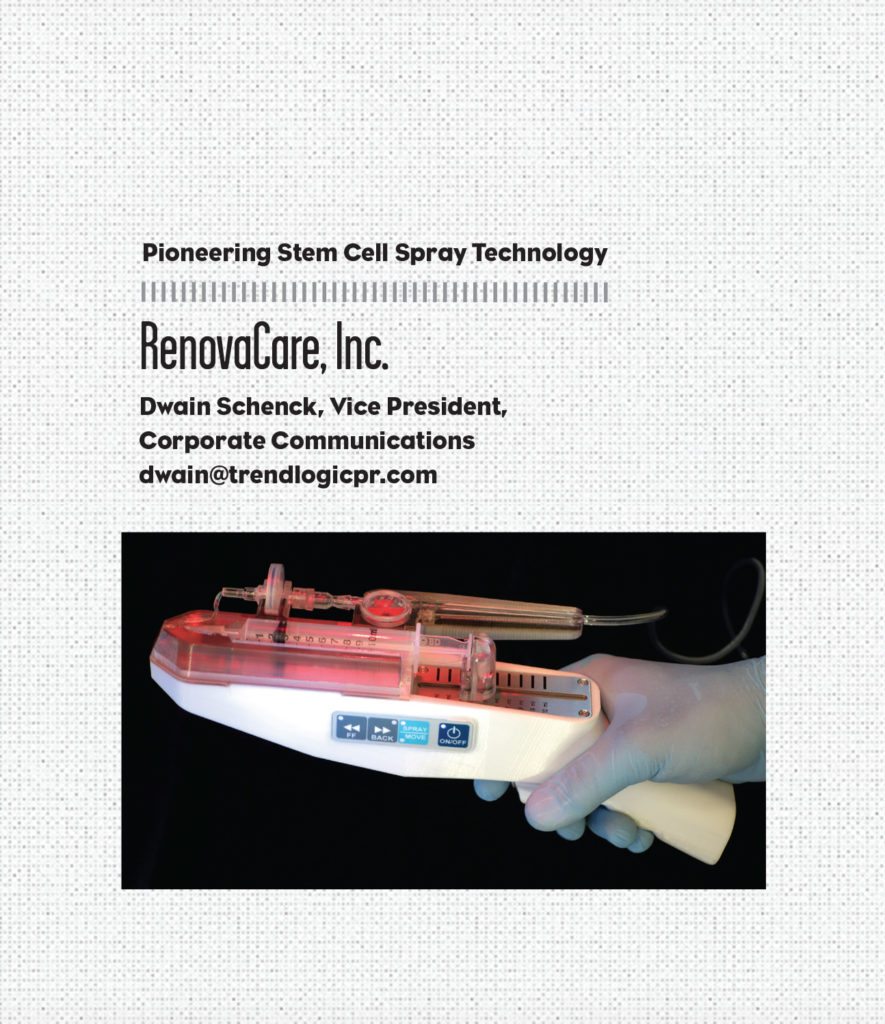
RenovaCare, Inc. is vying to be the first company to introduce an innovative burn treatment medical device called the SkinGun—a technology not entirely inspired by the iconic TV program Star Trek, but the aesthetics of the device’s design undoubtedly shares a distinct similarity to Captain Kirk’s particle-beam gun.
The functionality of the SkinGun is the result of years of stem cell research and next-generation bio-medical engineering. RenovaCare, which began its current operation as a biotechnology company three years ago, developed its flagship technology, the CellMist System and its patented SkinGun, to gently spray a liquid suspension of a patient’s stem cells on to severe burns and chronic wounds for rapid, scar-free healing.
The short 90-minute procedure replaces today’s skin grafting methods by isolating a small postage-stamp size skin sample. Unlike traditional grafting, physicians forego having to surgically remove large sheets of healthy donor skin needed for stitching over a patient’s wound site.
Burn victims also heal with full mobility in their joints in a matter of days as a result of the SkinGun treatment. In contrast, skin graft patients can remain hospitalized for weeks and months at a time, suffering through excruciating surgeries and undergoing physical therapy that can sometimes last up to a year.
To date, 72 patients have been successfully treated with the SkinGun—45 in Pittsburgh, PA and another 19 patients were previously treated in Berlin, Germany using an early prototype of the device. An additional eight patients were subsequently healed in Berlin, with advancements to the technology.
Sentient
Adam Cossman, Partner, President
Sentient is an award-winning, digital-first, metrics-driven marketing agency focused on helping life science companies launch and market new digital products and services or bring existing brands effectively into the online space. The company’s goal, regardless of the engagement, is to help its clients build enduring relationships with their target customer segments while creating measurable results for their brands.
Sentient’s approach revolves around its results-driven methodology. This approach ensures that all digital programs are measured, analyzed, and evaluated based on pre-defined key performance indicators, which are then used to establish a credible return on market investment (ROMI) model for the brand. Sentient prides itself on telling clients what they will achieve before they invest—and then proving it after.
One of the aspects of the agency that allows it to deliver on this results-driven methodology is its ability to provide services for user experience design, technical proficiency, and digital media. Sentient’s team structure includes creative services, technology services, and digital media/analytics integrated together into a cohesive working model. This affords Sentient the ability to operate with an agile approach to marketing—or “phragilie” as the agency’s partners have coined for applying these attributes within the heavily regulated pharmaceutical space.
Sentient’s leadership plans to continuously advance their unique approach through proprietary technology solutions including their ROMI modeling application, proprietary data warehouse, and data visualization tools that provide the agency and their clients with real-time access to actual brand results. Anticipating continuous change, they also see their approach evolving over the next five years to leverage technology to implement more dynamic real-time adjustments to content and digital experiences based on user behaviors and interactions.