PM360’s Innovations Issue, established four years ago, serves as a comprehensive guide to our readers, providing a glimpse at the year’s most cutting-edge: Companies, Divisions, Startups, Products, Services and Strategies.
Here are our picks for the most innovative products of 2015, which include software, apps, programs and anything else designed to improve how those working in the industry do their jobs.
AppScript
IMS Health
Brian Clancy, Senior Product Manager, Consumer Solutions
bclancy@imshealth.com
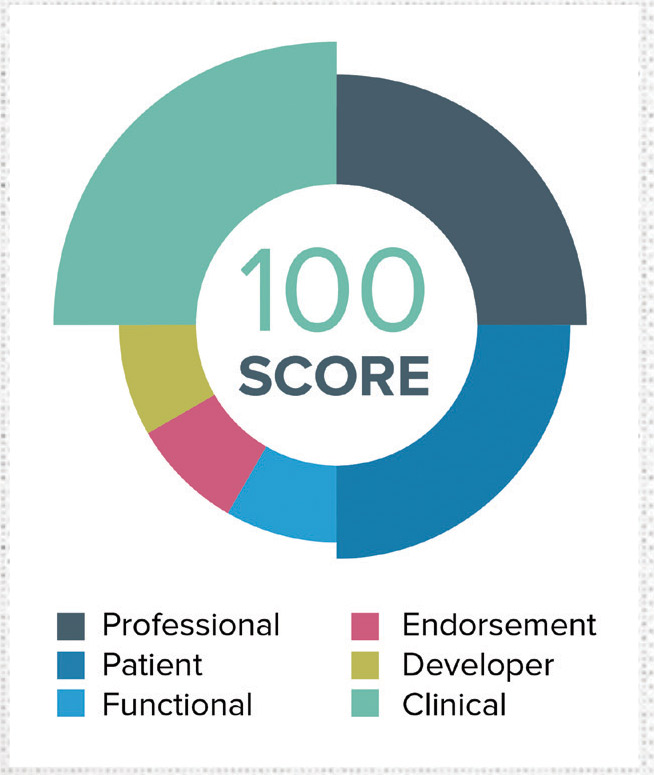
With more than 165,000 mobile health apps and devices in the marketplace, recommending the best solution can be overwhelming. IMS Health’s AppScript is the leading discovery and distribution platform for mobile health technologies. It’s a simple and effective mobile health tool that lets healthcare professionals recommend apps, connected devices and content to improve patient engagement, satisfaction and outcomes.
The AppScript team curated and characterized thousands of mobile healthcare apps, hundreds of connected devices, and thousands of pieces of educational content by condition and stage of the patient journey. With its proprietary algorithm, IMS Health has tested, rated and awarded a unique AppScript Score to more than 30,000 mobile health apps. The company’s proprietary IMS Health AppScript Score ranks apps and devices based on six primary metrics: Functionality, professional reviews, patient reviews, endorsements, developer trust ratings and clinical ratings.
With AppScript, prescribers can make their own mobile health formulary, customize each communication to reflect the institution’s brand and experience, and manage users from a small practice to a large institution. AppScript prescribers can see who opened their specialized AppScript prescription, send follow up messages to track patient satisfaction and usage, and securely connect with users via surveys.
The IMS Health AppScript team can also help pharmaceutical companies and mobile health app developers of all kinds to better understand the mobile health technology market, evaluate the clinical and economic value of mobile health interventions, and promote patient-facing mobile health interventions through B2B channels such as providers and payers.
ConferenceInsider
eHeathcare Solutions
John Burke, Chief Revenue Officer
jburke@ehsmail.com
eHealthcare Solutions (EHS) ConferenceInsider is a comprehensive, multichannel, three-month digital program that makes it easier for life science marketers to deliver a brand message in conjunction with a key healthcare industry conference in alignment with their target audience.
The ConferenceInsider program enables brands to stay top-of-mind with both conference attendees and non-attendees interested in relevant conference content. The program consists of live event coverage including: Speaker session highlights, poster sessions, video posts from Key Opinion Leaders (KOLs), daily polls and live tweets, each delivered on a brand-sponsored custom conference microsite hosted by Drugs.com, an exclusive EHS publisher partner. Customizable integrations of other EHS solutions with the ConferenceInsider, such as their Test Your Knowledge diagnostic challenge quiz game for healthcare professionals (HCPs), are also available.
EHS then leverages its exclusive healthcare professional publisher network, including more than 75 of the most respected online medical journals, healthcare professional and medical society websites to reach the desired specialty audience. Through this approach, EHS enables healthcare marketers to maximize value through a variety of digital engagement tools, including branded, custom emails to list-matched, authenticated HCPs, and geo-medically targeted advertising to drive traffic to the brand-sponsored ConferenceInsider microsite before, during and after the chosen event.
EHS ConferenceInsider launched in May 2015, and all ConferenceInsider programs to date have exceeded the reach and engagement expectations of the brand partner. This service is demonstrably helping pharmaceutical brands engage targeted HCPs with relevant, timely content that keeps physicians and prescribers up to date with the latest advances in treatments and technologies for their profession.
ContentShare
Compas, Inc.
Brandy Colangelo, Director, Buying Services and Deliverables
bcolangelo@compas-inc.com
Late this year, Compas, Inc., a leading media buying firm, launched ContentShare, a proprietary service for the strategic distribution of audience-specific clinical and pharmaceutical client content in both print and digital formats. By harnessing the power of key clinical and pharmaceutical content, ContentShare is able to offer a content planning strategy that can proactively drive a brand’s clinical content to their target audiences and provide relevant healthcare material for increased brand awareness and prescription lift.
Recently, many in the pharma industry have shied away from this kind of content and medium because of fears that it will violate the Sunshine Act. But according to CMI/Compas’s Media Vitals 2015 research study, the greater risk to pharma may be not distributing reprints or other content to healthcare professionals. The research revealed that 90% of healthcare professionals actually think it is useful to receive medical journal article reprints from pharma. And now ContentShare allows clients to use and track this type of content in a way that’s compliant with the Sunshine Act—providing another powerful engagement tool that clients can use to move the promotional needle.
ContentShare made its debut at ProcureCON Pharma in November, where one client who attended noted: “This is a great way to ensure content is getting in front of the right HCP, especially HCPs that are not being reached by sales reps!” Another client attendee noted: “I like how this provides not only cost efficiencies but a customized approach to deliver key content.”
GateKeeper
PSKW
Chris Dowd, Executive Vice President, Market and Product Development
cdowd@pskw.com
The federal Anti-Kickback Statute (AKS) stipulates that in a claim scenario in which a person is covered by a government health plan and benefits are paid by the government, the use of pharmaceutical co-pay cards or patient savings cards is not permitted. Any violation of this provision could result in significant fines. Sadly, identifying government beneficiaries isn’t as easy as it sounds.
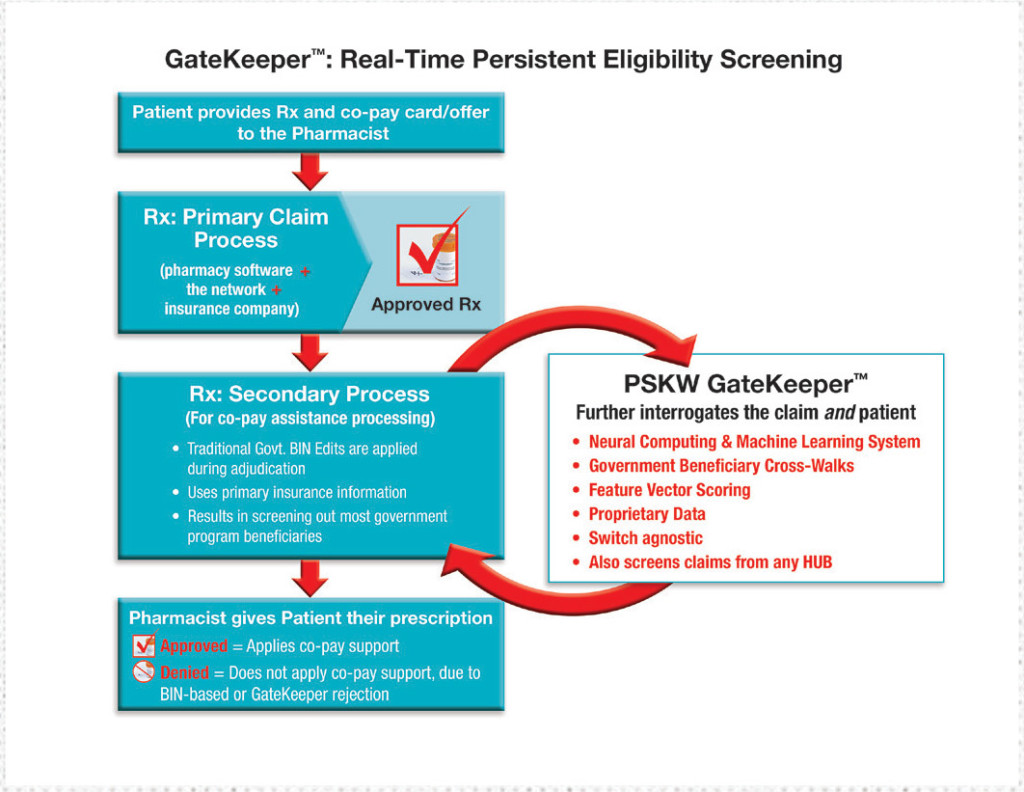
In their primary effort to comply with this AKS provision during each pharmacy claim adjudication event, pharmacies, manufacturers, adjudicators, and co-pay program providers have thus far relied on one or more of several plan identifiers listed on the patient’s insurance card. These include the Plan ID, Payer ID, BIN (Bank Identification Number), PCN (Processor Control Number), and Rx Group number. Unfortunately, these insurance-card identifiers (used alone or in combination) are an inadequate means by which to screen for government beneficiary status.
In 2015, PSKW developed GateKeeper in partnership with EagleForce Health, the advanced technology firm responsible for the proven model used by several of the most security-sensitive agencies on earth. GateKeeper helps companies comply with the AKS provision without relying on inadequate BIN/PCN/Group ID data. Instead, GateKeeper uses advanced technology to collect billions of person-specific data points from nearly 1,000 disparate data sources, sorts and collates those data points to accurately ascertain whether a specific individual is participating in any government-run health insurance program, and then blocks ineligible individuals from receiving co-pay assistance.
iSpecimen’s Virtual Marketplace
iSpecimen
Kiran Ganda, Director of Marketing
kganda@ispecimen.com
With the advent of personalized medicine, biomedical research is booming—magnifying the need for human biospecimens on which to conduct the research. But finding qualified human biospecimens has long been a problem. In fact, in a 2009 National Cancer Institute study, 81% of researchers said they limit the scope of their work due to the shortage of quality biospecimens. Fast forward to today, when the need is even greater, and the extent of the problem is palpable. And yet each year millions of human biospecimens are destroyed when clinical testing is complete—but they could be put to good use.
iSpecimen created a new paradigm for specimen procurement through which technology easily interfaces with health information systems at hospitals and labs to watch the flow of soon-to-be-discarded specimens and their associated data. When a match to a researcher request is made, specimens are picked, packed and shipped in an automated, HIPAA-compliant, efficient manner to researcher customers.
This is the first specimen procurement process leveraging technology and health system data, including Electronic Medical Record data, allowing scope, scale and detailed annotation. Through iSpecimen’s virtual marketplace, researchers can submit extraordinarily specific requests including solid and liquid specimens from patient cohorts that span different demographics, diagnoses, medications and procedures. The technology also incorporates de-identification and consent processes to address protected health information and patient preferences.
As a measure of success, since launching officially in May, iSpecimen is already implemented in dozens of hospitals and labs nationwide and delivering to a wide range of customers, including pharmaceutical, diagnostic, biotech and academic organizations.
Medtronic CardioInsight ECVUE Sensor Array Vest
Nottingham Spirk
Bill Nottingham, VP Business Development
bnottingham@nottinghamspirk.com
When CardioInsight, a medical device company launched by the Case Western Reserve University Technology Transfer Office (CWRU TTO), invented a breakthrough technology to improve diagnosis and treatment of electrical disorders of the heart, Charu Ramanthan and Ping Jia decided to bring in Nottingham Spirk as a development partner for their creative engineering and design skills and success in getting products to market.
The device in question is a vest that allows an electrocardiologist to view exactly what is happening in a fully 3D virtual environment specific to that patient’s anatomy, completely non-invasively, in real time. The system can be used for precise diagnosis to determine what treatment plan is most appropriate for the patient, whether it be lifestyle changes, pharmaceutical treatment or surgical intervention. And if a surgical treatment is determined to be the best course of action, it can also be used to guide the procedure.
CardioInsight’s original prototype had more than 250 electrodes, weighed approximately 20 pounds, and resembled a medieval-looking garment strewn with wires. The teams worked collaboratively to create a lightweight, disposable vest with 250 plus electrodes printed directly onto the vest substrate. This version was comfortable, easy to put on, could fit a variety of body types and allows the mobility needed to navigate the patient through the multiple steps of the entire 8-hour procedure throughout the hospital. The system also enables more efficient use of hospital resources by decreasing the time the patient spends in each department.
The final result: The CardioInsight ECVUE Sensor Array vest, which received a Gold Edison Award and was an IDEA Finalist. In 2015, Medtronic acquired CardioInsight for about $93 million.
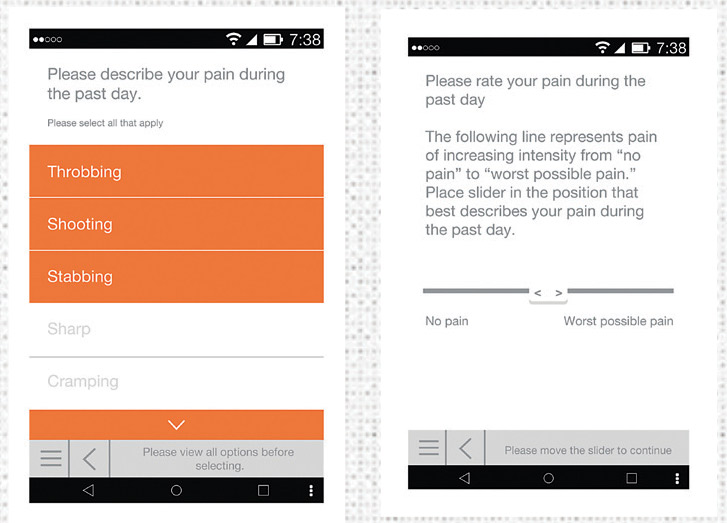
Mobile Survey App
Kantar Health
Brian Mondry, Global Head of Digital Innovation
brian.mondry@kantarhealth.com
In 2015, Kantar Health got closer to the chronic pain experience through a mobile pain diary study with rheumatoid arthritis patients. A mobile version of a validated pain scale plus patients’ pictures with descriptive captions enabled the company to understand the chronic pain patient experience—in an emotional as well as a clinical context. The combination of words and visuals enabled patients to share their true reality. Not only ice wrapped ankles, but a mom unable to play with her baby and overflowing hampers and dishwashers because patients are in too much pain to do simple household chores.
But mobile now goes way beyond the smartphone as advances in mHealth technology allow for the passive collection of accurate biometric data. Combined with data actively collected through surveys and other means, this provides a more holistic view of the healthcare consumer and helps identify the levers that influence attitudes, behaviors and health outcomes.
In response to this, Kantar Health developed a mobile survey app that is able to passively collect biometric and activity data from a wide range of mHealth devices. So in addition to survey and multimedia data, the app adds another layer of information through accurate biometric data. In the pain category, mobility data may provide a guide to whether certain levels of activity trigger a pain episode. Or it can be used to monitor how users of a client’s pain solution fare in real-world situations. This can have major implications for studies conducted around compliance and efficacy.
onevoice
Clear Pharma
Dan Donovan & T Anthony Howell, Co-Founders, Clear Pharma
onevoice (www.onevoice.world) is a super-charged multi-stakeholder engagement platform specifically designed for rare disease communities. It provides a genuine opportunity to introduce a truly patient-centric offering allowing a sponsor to become a valued member of the rare disease community. By doing so, a sponsor will accelerate understanding and awareness of rare diseases by connecting all stakeholders, potentially resulting in a significantly decreased reliance on “feet-on-the-street” sales mentality.
Each onevoice software platform goes narrow and deep, one rare disease at a time, providing true value to the full stakeholder community, offering emotional support, curated knowledge and an array of clinical trial services. Clear Pharma’s vision is to build hundreds of onevoice platforms creating the backbone for rare disease communities around the world—allowing them to accelerate understanding of each disease and an ability to collaborate to improve disease outcomes. Additionally, the power of the data collected within and across onevoice platforms can be harvested and shared with the community to greatly expand understanding of both the rare disease and the patient journey.
In creating onevoice, Clear Pharma sought to connect intelligence, data and people to make complex searches easy and to provide users with curated information customized to their learning proclivities and information needs in a variety of ways, including “encouragement power” through gamification; predictive information sharing; linkwheel navigation of social media, blogs, etc.; and a clinical trial wizard which provides a report of probability for inclusion in clinical trials.
The first onevoice platform will launch in the first quarter of 2016. It is co-sponsored by a pharmaceutical company, in partnership with a nonprofit foundation, who will make it available to the rare disease community for free.
Patient Mobile Connect & Digital Exam Room Wallboard
ContextMedia:Health
Ashik Desai, EVP of Business Growth and Analytics
ashik.desai@contextmediahealth.com
ContextMedia:Health recently launched two innovative solutions: Patient Mobile Connect and the Digital Exam Room Wallboard.
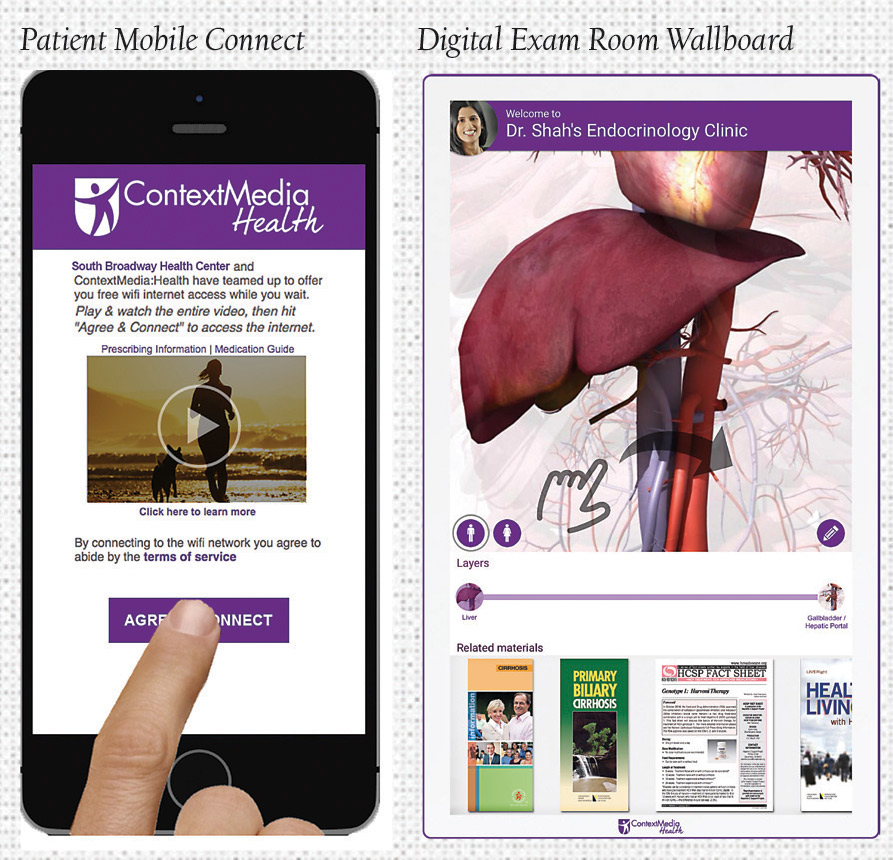
Patient Mobile Connect offers the first DTC product at the point of care to reach patients in their doctor’s office, on their mobile device. When patients select their doctor’s WiFi network, they receive a digital banner or branded video spot, which is viewed in its entirety. Each option allows access to additional educational information and guarantees 100% visibility throughout the entire office. Patient Mobile Connect takes the guesswork out of targeting the right patients on their mobile device with custom messaging according to WiFi range.
Meanwhile, the Digital Exam Room Wallboard captures patient focus when and where it matters most: Within the exam room. After the success of the Exam Room Tablet, the addition of the Digital Exam Room Wallboard to ContextMedia:Health’s suite ensures that relevant information is delivered to patients in the exam room at all times. The 32-inch interactive flat screen offers the opportunity to promote brand messages in a high impact, visual way, by bringing pre-approved assets to life inside the exam room. It creates an interactive experience for patients, delivering digital brochures, infographics and promotions that patients can interact with and forward to their personal emails for post-visit engagement.
Notably, the Digital Exam Room Wallboard also includes an interactive physician mode, which allows physicians the ability to educate patients with detailed, 3D responsive anatomical diagrams that they can mark to illustrate aspects of patient illness, educate on treatment options, and communicate important treatment information to improve retention.
Predictive Multichannel Targeting
Liquid Grids
Malcolm Bohm, President & CEO
malcolm@liquidgrids.com
Liquid Grids was founded in 2010 with the mission of simplifying the complexity of online disease dialogue and making social health intelligence strategically actionable. In 2015, Liquid Grids extended its capabilities beyond intelligence to predictive multichannel targeting. To do this, the company indexed the most relevant destinations across the World Wide Web where consumers are interacting on health topics.
For each result, the company’s technology is deriving all available statistics about each destination: Content keywords and phrases, posts and comment strings, visitor rates, costs per click, competitive website information, etc., and then calculating propensity scores for each destination. The result is a rank ordering of the World Wide Web, including the social networks, for health interactions.
Liquid Grids also developed a breakthrough in the application of computational linguistics. The technology inspects content across the Web to surface the unique context of consumer dialogue. In this example post: “I have a very painful migraine and need to be in a dark room for the rest of the day,” the “dark room” is identified as the context linked to the migraine diagnosis and pain symptom.
This combination of highly precise disease dialogue context and predictive targeting parameters is used to develop the best ad content, creative and placement strategies across premium search, display and programmatic buying. This extension of context intelligence into ad tech targeting helps ensure the pharmaceutical industry’s advertising and messaging reaches the right audiences, with the right information, in the context of their interactions on the Web and their disease status.
Reltio Cloud
Reltio
Ramon Chen, Chief Marketing Officer
ramon.chen@reltio.com
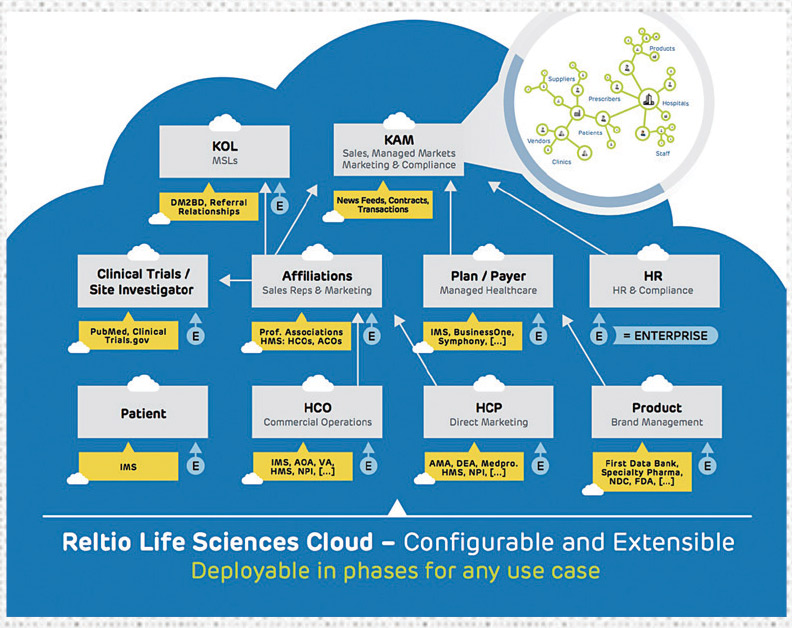
Reltio Cloud is a modern data management platform combined with data-driven applications specifically designed for the healthcare and life sciences industry. One of Reltio’s goals is to break down the barriers between IT and frontline business users by ensuring a reliable master data foundation upon which data-driven applications with mobile, easy-to-use interfaces can be delivered to sales, marketing and compliance teams. These applications provide contextual insight based on the goals of each user, giving them recommended actions for proven ROI. Some of Reltio Cloud’s latest offerings include:
Reltio for Key Account Management: This first of its kind application brings all the data together for KAMs to be successful. No longer will they have to rely on CRM systems, combined with spreadsheets and information scattered across multiple tools and applications. This offers a single interface for collaboration and insight across all teams within a life sciences enterprise.
Reltio for Key Opinion Leader Management: Similarly, this brings together data across multiple data sources to deliver real-time ranking, rating and collaboration among marketing and sales teams to identify and manage KOLs for any purpose. Built-in analytics and machine learning algorithms ensure the most objective selection based on continuously refreshed inputs and criteria.
Reltio for Managed Markets: Brings together a complete view of HCPs, patients, MCOs, IDNs, plans, payers, ACOs, products, employees and more. Similar to all Reltio data-driven applications, this solution not only helps pharma work better with payers and providers, but also is being used as a path to better understand patient data, with a view towards patient centricity.
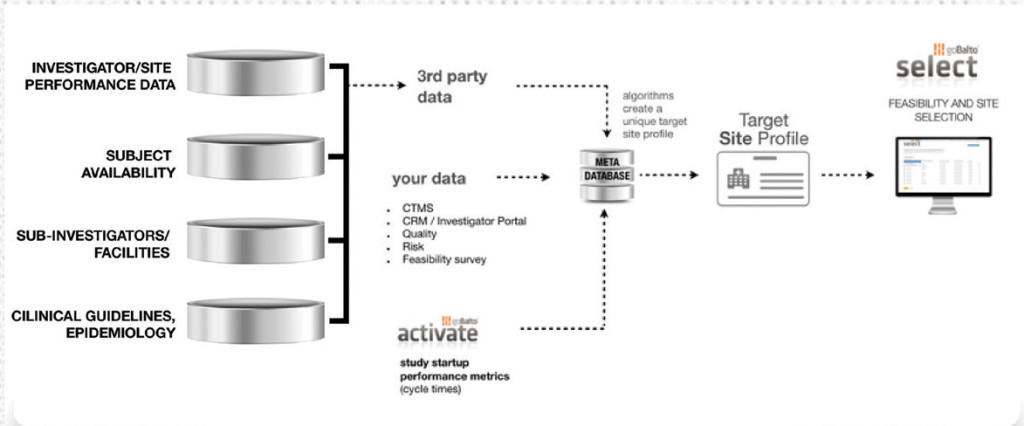
Select
goBalto
Michael Thompson, Director of Business Development
mthompson@gobalto.com
The life sciences industry has recognized study startup as one of the worst performing areas in clinical trials—80% of trials fail to meet enrollment timelines and up to 50% of research trial sites enroll one or no patients. According to Cutting Edge Information, 72% of studies run more than one month behind schedule, with sponsors standing to lose up to $10 million for each day that the trial delays a product’s development and launch.
Many organizations struggle to control costs and resources associated with ramping up clinical trials. Current startup processes involve Excel trackers which don’t support collaboration since documents must be faxed or sent by courier, and because reporting is manual and teams don’t have visibility into real-time study status.
goBalto develops next-generation solutions that simplify how clinical trials are started. Their flagship product, goBalto Activate, is a software-as-a-service (SaaS) clinical research platform that enables clinical trial sponsors and clinical research organizations (CROs) to track milestones and provides document workflow management capabilities in a transparent, regulatory-compliant, and user-friendly way.
This year, goBalto released Select, which is an end-to-end workflow solution designed to help sponsors and CROs avoid non-active and non-enrolling sites by addressing the data pain points associated with dealing with disparate data sets and a lack of institutional memory by eliminating manual processes for site selection. Select combines internal and external data sources to create a complete target site profile and enable an evidence-driven site selection processes.
Clients who have used goBalto products have been able to cut more than 30% of the time required to activate sites for clinical studies.
Trumenba Augmented Reality Starter Kit
Artcraft Health
Shauna Aherne, Director, Market Strategy
saherne@artcrafthealth.com
Educating and empowering behavior in adults can be challenging, but it is even more so in teens. Artcraft Health developed an integrated starter kit that incorporates Augmented Reality to engage and enrich a teen’s interaction with the resource, and motivates the teen to get vaccinated for meningitis B. The interactive starter kit visualizes the three key steps to help protect against meningitis B: START the journey; STAY the course; SHARE the facts. Artcraft Health leverages Augmented Reality in the app as a unique and competitive differentiator to reach teens on health education in an innovative way.
Once downloaded, teens hold their phones over three separate Augmented Reality “targets” and experience a 3D view of video clip of a meningitis B survivor sharing her story; view animations of a teen feeling ill (from onset of vague flu-like symptoms to death can occur in little as 24 hours) and common items that may spread the disease (sharing cups, sharing lip gloss, eating from the same bowl of popcorn). In addition, the branded app contains a dose tracker (it requires three doses), disease state education, and sharing functionality.
Augmented Reality has been around for quite some time; however, recent advances now place full functionality on mobile devices, making it an effective and interactive tool that expands, extends and provides for a memorable user experience—one that teens share with their friends and loved ones.
Since launch, the app has been downloaded 1,600 times and has been given a five star rating in the Google Play store. Promotional efforts continue, building awareness and driving traffic to the app and its rich visual, interactive and relevant content.
Veeva OpenData
Veeva Systems
Jason Wreath, Vice President, OpenData Products & Marketing
jason.wreath@veeva.com
For commercial teams looking to create meaningful engagement with their customers, nothing is more important than having the right customer data. Off-the-shelf data feeds can help, but it takes attention and effort to keep everything up-to-date. That’s because details such as addresses, affiliations and license status can change frequently, causing data dissonance and compliance risks.
In March 2015, Veeva Systems launched Veeva OpenData, a new approach to customer data that is open, easy and global. Veeva OpenData delivers millions of records for healthcare providers, organizations, affiliations, compliance, and email data available across major life sciences markets worldwide. Veeva OpenData ensures quality and completeness through rigorous, automated processes and steward-led validation. Data change requests are a standard service, and most are completed in under a day. With Veeva OpenData, commercial teams can:
• Improve targeting: Identify customers and personalize interactions, using consistent, high-quality customer data.
• Improve compliance: Engage in the right discussions, accurately track and report aggregate spend, and ensure compliant activity.
• Engage across multiple marketing channels: Email, phone, fax, mail or in person, Veeva OpenData provides the necessary and correct data to connect with customers in a variety of ways.
• Lower costs: Veeva’s unique “All-In” pricing approach keeps costs low and predictable for data that can be used without restriction or surprise fees.
Additionally, Veeva established the Veeva OpenData Partner Program—a grass-roots ecosystem of like-minded organizations that understand the importance of making it easy for life sciences companies to have unlimited use of their customer data. Working with dozens of industry leaders across the globe, companies can integrate and consume their data freely.