Discoveries/Innovations: Nanotech Microchip Detects and Monitors Type 1 Diabetes
Researchers at Stanford University have invented a portable microchip-based test for diagnosing type 1 diabetes that can potentially improve patient care and help researchers better understand the disease. Patients with type 1 diabetes may benefit from the nanotech microchip’s ability for fast detection and the possible halting of the autoimmune attack on the pancreas, thereby preserving some of the body’s ability to make insulin.
The microchip uses no radioactivity, produces results in minutes, and uses smaller volumes of blood than older tests done with a finger prick.
This test also allows doctors to quickly and cheaply track patient’s auto-antibody levels before they show symptoms. With an expected cost of about $20 to produce, each chip can be used for upward of 15 tests. It may also allow the first broad screening for diabetes auto-antibodies in the population at large.
Stanford University and the researchers have filed for a patent on the microchip and the researchers also are working to launch a startup company to help get the method approved by the FDA and bring it to market, both in the United States and in parts of the world where the old test is too expensive and difficult to use.
DC Dispatch: Medicare Revises Hospice Rules
After a 2012 investigation by the Department of Health and Human Services, Medicare officials are modifying rules intended to prevent the agency from paying twice for the same prescriptions for seniors receiving hospice care. Prior to the revision, hospice patients or their families could not fill prescriptions through their Part D drug plans until first confirming that the prescriptions were not covered by hospice providers.
Medicare announced that the rules would be revised so that additional authorization would be required for only four types of medications: Pain relievers, anti-nauseants, laxatives and anti-anxiety drugs that are “nearly always” considered hospice related. According to Medicare spokesman Raymond Thorn, the rules adjustment gives beneficiaries enrolled in hospice continued access to their medications while balancing recommendations by the Inspector General.
Med Device: Microchip Shows Efficacy in Osteoporosis Patients
A recent study involving MicroCHIPS, an implantable controlled drug delivery device, yielded promising results in patients with osteoporosis. Women diagnosed with osteoporosis between the ages of 65 and 70 received daily doses of the marketed drug, teriparatide, through the microchip. The drug released from the implanted microchip showed similar measures of safety and therapeutic levels in blood as compared to the standard, recommended injections of teriparatide. The programmable implant delivered the drug at scheduled intervals with similar consistency and efficiency over 12 to 24 months, according to the study. Patient surveys found the microchip device was well tolerated, with most saying they would repeat the implant procedure.
Therapeutic Talk: New Developments in Parkinson’s Disease Drug Development
Good news for the one million Americans and the more than five million people worldwide diagnosed with Parkinson’s! With no available disease modifying therapy able to treat the cause of Parkinson’s or delay the disease progression, a new clinical development landscape offers hope for future potential new therapies.
Currently, 33 drugs are in later stage development for Parkinson’s, according to the Raise Awareness for Parkinson’s Drug Development on Moving Day report from Citeline. The majority of those in later development (15 total) are in the treatment category of “Reformulation of Approved Parkinson’s Drugs.” In this category, drugs are typically delivered as monotherapy and aim to decrease the “off-time” of treatment and the effects of prolonged treatment.
The most novel of the potential new treatments are likely to come from one of three categories: “Cell/Peptide Therapy,” “Natural Product” and “Gene Therapy.” This grouping represents the only treatments for possibly modifying the disease and delaying disease progression with currently six drugs in Phase II and one in Phase III.
The third category, with a high subset of drugs, are focused on decreasing levodopa-induced dyskinesia. Five drugs are at Phase II, and one at Phase III. The figure below provides a snapshot of the top five companies sponsoring the drugs/trials in each of the three major treatment categories.
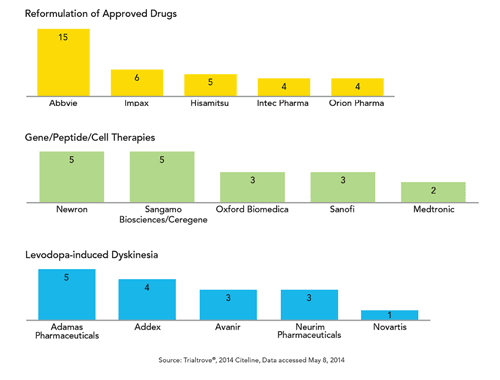
So far the treatments in the Reformulations of Approved Drugs category have demonstrated the most success in trails with 72% of completed efficacy trials meeting their endpoint. Meanwhile, there is a 57% efficacy success rate of treatments with the new chemical entities for levodopa-induced dyskinesia. And the novel Gene/Peptide/Cellular therapies group shows 50% efficacy success.
FDA Update
First Biologic for Cervical Cancer Increases Survival Rate
Genentech received approval for the new biologic medicine, Avastin, when used in combination with chemotherapy, for the treatment of women with persistent, recurrent or metastatic cervical cancer. In a National Cancer Institute study conducted by the Gynecological Oncology Group, the Avastin and chemotherapy combination improved overall survival by a median 16.8 months, as opposed to 12.9 months with chemotherapy alone. The new biologic is approved in the U.S. to treat five distinct tumor types.
Recent Approvals
SynCardia Systems’ SynCardia temporary Total Artificial Heart with SynHall Valves recently received approval as a bridge to transplant for people suffering from end-stage biventricular heart failure. The FDA expanded indications for Pfizer’s and Bristol-Myers Squibb’s oral anticoagulant Eliquis to treat deep-vein thrombosis and pulmonary embolism—and also lower the risk or the recurrence after treatment. Triumeq (a combination of abacavir, dolutegravir and lamivudine) developed by ViiV Healthcare, a joint venture of GSK, Pfizer and Shionogi, received approval as the first single-pill regimen containing dolutegravir for the treatment of HIV.
Expanded openFDA
The FDA added to its openFDA initiative with a recently released API that allows users to search for adverse event data on medical devices as far back as 1992. Among other things, the API helps identify classes of devices that may be associated with certain adverse events and provide developers and researchers with insights to create innovative products that protect and promote public health.






