Doctor Docs
Physicians Say Yes to Brand Requests
When patients bring up a brand-name drug, doctors do more than listen—they pull out their prescriptions pads. In a 2009 national survey of approximately 1,900 U.S. physicians, 37% said that they often or sometimes prescribed a brand-name drug—even if an appropriate generic substitute was available—simply because patients requested it, according to the results recently published in JAMA Internal Medicine. This was especially true for respondents who received industry-sponsored food or beverages in the workplace as well as free drug samples. However, there did not appear to by any correlation with respondents who received industry gifts or reimbursement for travel expenses, or with those who served as industry-paid speakers or consultants. In terms of specialties, internists and psychiatrists were the most likely to honor requests for a brand name. And physicians in practice for over 30 years were more likely to fulfill the patient’s desire for a brand than physicians with less service time.
“The good news is that 63% of physicians indicated they never or rarely prescribed a brand-name drug instead of an equivalent generic simply because of a patient request. However, our data suggest that a substantial percentage—37% or about 286,000 physicians nationally—do meet those requests,” says Eric G. Campbell, HMS professor of medicine at Mass General, who led the study. “Since generics are from 30% to 80% cheaper than the brand-name versions, that would represent a significant source of unnecessary health costs.”
Psoriasis Patients Go Social
Social media can be a great way for patient groups to connect with each other for support and psoriasis patients are no exception. Manhattan Research and the online patient community network, Inspire, collaborated on a survey to better understand how psoriasis patients use social media. Here are a few highlights from the survey of 317 patients:
70% of those surveyed use any type of online community (social network, message board, blog, etc.) at least several times a week, while 44% visit a psoriasis-specific social media site multiple times per week.
84.5% visit psoriasis social media sites to read how others have managed their conditions, 77.6% are seeking tips and advice they can’t find elsewhere, 65.9% are trying to find other treatment options, 60.6% are looking for reviews of prescription drugs, and 45.5% wanted to connect with others for emotional support.
34.1% use these communities on a long-term basis with regular visits, 24.6% only visit when a medical situation occurs, and 12.9% rarely return after they first signup.
69.4% only belong to one patient community (TalkPsoriasis) while 30.6% belong to more than two.
DC DISPATCH: HHS Finally Announces Revised HIPAA Provisions
In 2009, the passage of the “Health Information Technology for Economic and Clinical Health Act” or the HITECH Act, called for a revision in the HIPAA Privacy Standards that would restrict certain communications by pharmacies and other providers regarding drugs and medical devices. Well, the Office for Civil Rights of the U.S. Department of Health and Human Services (HHS) has finally announced the final omnibus rule.
The rule revision expands a patient’s rights in a number of ways including enhanced access to his/her electronic medical record. Patients also have more power over who gets to see their information. When paying via cash, they can prevent their provider from sharing information about their treatment with their health plan. And in what may be the most relevant revision to pharma marketers, patient authorization is now required before protected health information can be used for any paid communications that recommend a product or service to the patient.
For instance, pharma companies would typically pay pharmacies to send messages to patients recommending a switch to an alternate therapy. Well, not anymore. However, prior authorization is not required for communications about any drug already prescribed, so refill reminders and adherence messages are still fair game. Another plus for patients: the sale of an individual’s health information is prohibited without their permission. But one bonus for researchers: the final rule streamlines a patient’s ability to authorize the use of his/her health information for future unspecified research purposes. To read the final rule in its entirety, visit bit.ly/HIPAARule.
SALES SECTOR: Quarter Four Sales Update
Companies are starting to release their quarter four (Q4) earnings reports and the results are…mixed. Humira certainly gave Abbott a reason to smile with a 31% increase over the same period last year in U.S. sales—bringing in a total of $2.7 billion. AndroGel also surged a whopping 41% while brands such as Trilipix/TriCor (-51%) and Kaletra (-17%) took hits. Overall, Q4 net sales increased 4.4% to a total of $10.8 billion while Abbott’s net profit in Q4 was only $1.1 billion—a 35% decline. However, the company did have to pay $265 million in charges following the spinoff of AbbVie into its own research-based biopharmaceutical company.
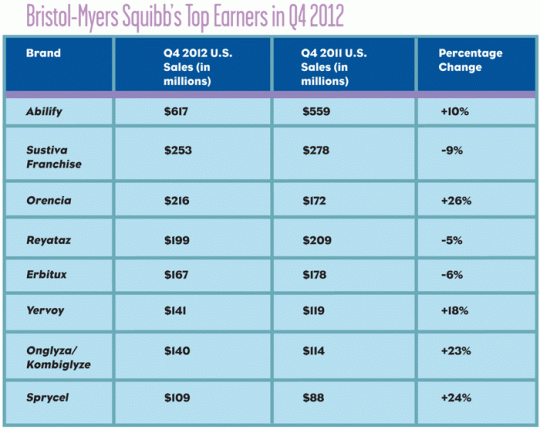
Meanwhile, Bristol-Myers Squibb posted a 23% decline in Q4 total sales, which should really come as no surprise considering both Plavix and Avapro/Avalide went off patent. Plavix sales in the U.S. took a nose-dive, declining 99% over the same period last year while Avapro/Avalide plummeted 86%. The good news is, excluding Plavix and Avapro/Avalide, net sales actually grew by 13% compared to Q4 2011. The real cherry on top? Net earnings in Q4 actually increased from $852 million in 2011 to $925 million. Orencia, Sprycel and Onglyza/Kombiglyze were BMS’s biggest gainers in sales (see table below).
Baxter was full of good news with a 7% increase in Q4 U.S. sales and a net income of $494 million after bringing in $463 million in the prior-year period. BioScience revenues totaled $1.7 billion—a 7% increase—which the company attributes to robust growth for Advate and Gammagard Liquid, especially in the U.S., though individual figures for the drugs were not released.
FDA UPDATE:
First of its Kind
The FDA recently approved a few new treatment options, the likes of which we have not seen before. Unlike current flu vaccines, Protein Sciences Corp.’s Flublok does not use the influenza virus or eggs in its production. Instead, its novel manufacturing technology—using an insect virus expression system and recombinant DNA technology—allows for production of large quantities of the seasonal flu vaccine. Fulyzaq (crofelemer), marketed by PharmaDerm, is the first antidiarrheal approved for HIV/AIDS patients taking antiretroviral therapy. Janssen Therapeutics’ Sirturo (bedaquiline) is the first treatment for multi-drug resistant pulmonary tuberculosis (TB), which occurs when TB becomes resistant to the drugs most commonly used to treat it. Finally, Sobi’s Kineret is the first and only approved treatment for neonatal-onset multisystem inflammatory disease (NOMID), the most severe form of cryopyrin associated periodic syndromes (CAPS).
New Indications
Meanwhile, some old drugs have learned a few new tricks. The new indication for Genentech’s Avastin will allow people who received Avastin plus an irinotecan or oxaliplatin containing chemotherapy as an initial treatment—(first-line) for mCRC—to continue to receive Avastin plus a different irinotecan or oxaliplatin containing chemotherapy after their cancer worsens (second-line treatment). Allergan’s Botox injection can now be used to treat patients with an overactive bladder that does not respond to conventional medications. And Novartis has two drugs with new uses. Exjade (deferasirox) is now also a treatment for patients ages 10 years and older who have chronic iron overload resulting from a genetic blood disorder called non-transfusion-dependent thalassemia (NTDT). And Gleevec (imatinib) can now be used to treat children newly diagnosed with Philadelphia chromosome positive acute lymphoblastic leukemia.