Med Device: New Microscope Maps the Brain Down to Each Neuron
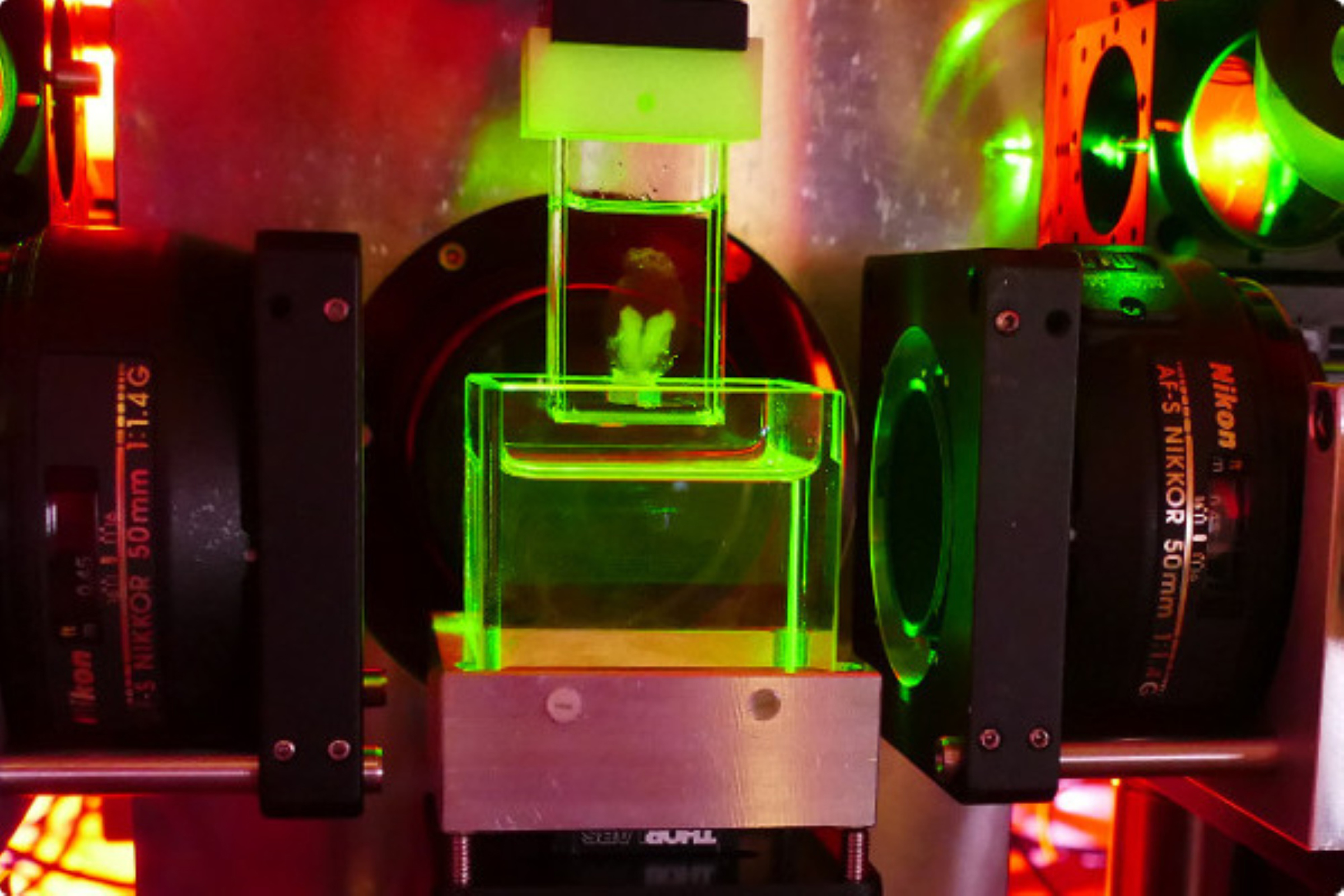 The new mesoSPIMs microscope is able to create 3D images of the brain and other small organs in never before seen detail. This new generation of microscope captures these images faster than any before, creating renditions of brain tissue that contain each individual neuron. MesoSPIMs will be an extremely insightful tool for researchers who need to understand brain and spinal cord organization. This knowledge is especially powerful for those working to restore movement after paralysis or investigating the neuronal networks involved in cognition or drug addiction.
The new mesoSPIMs microscope is able to create 3D images of the brain and other small organs in never before seen detail. This new generation of microscope captures these images faster than any before, creating renditions of brain tissue that contain each individual neuron. MesoSPIMs will be an extremely insightful tool for researchers who need to understand brain and spinal cord organization. This knowledge is especially powerful for those working to restore movement after paralysis or investigating the neuronal networks involved in cognition or drug addiction.
MesoSPIMs, short for mesoscale selective plane-illumination microscopes, are light-sheet microscopes. This means a beam of light slices through the tissue sample, mapping the internal structure by measuring light refraction. This is done over and over until the entire tissue sample is measured (without being physically sliced through) and a 3D image can be rendered. The amazing part: This is all done in seconds. Datasets produced by standard light-sheet microscopes are very large and time-consuming to analyze, which was previously a problem for those needing to visualize large samples.
Besides conquering this time challenge, mesoSPIMs will overcome access issues on its open-hardware microscopy platform. The mesoSPIM Initiative was started by Dr. Fabian Voigt of the Brain Research Institute, University of Zurich. His aim is to share this cutting edge technology with the world. “We created the open-source mesoSPIM Initiative to share the latest developments in microscope instrumentation and software with the imaging community. Anyone seeking high-quality anatomical data from large samples now has the information they need to build and operate their own mesoSPIM,” said Voigt. There are currently seven mesoSPIMs in operation across Europe, and several more under construction.
Patient Pages: Women with Endometriosis: Hormone Shopping
Women with endometriosis often depend on hormone therapies to manage their symptoms. Unfortunately, finding the right treatment and managing their side effects is often a long and difficult journey. A new Health Union Endometriosis In America 2019 survey of over 1,200 women found that four out of 10 have had to try five or more different hormone therapies in an attempt to better manage their condition.
Most of these patients have had to start their treatment journey by seeing six or more different doctors before even receiving an endometriosis diagnosis. They are also more likely than the subset of women who have used one to four hormone therapies to describe their endometriosis as severe and have undergone three or more laparoscopies. These patients indicated that they feel like they have tried everything possible to manage their condition. However, their symptoms are still not controlled.
In a landscape with numerous treatments to choose from and an arduous process for determining which one is best for each individual, pharma marketers should differentiate their products in a way that helps patients overcome their negative associations. In the meantime, online health communities like Health Union’s Endometriosis.net offer safe places for people living with chronic health conditions to connect with others and find information, validation, and support.
DC Dispatch: Marketing Cannabis Amidst Vaping-Related Illnesses
 The recent lung illnesses that may be linked to the Vitamin E component in nicotine vaping oils has been garnering much public and governmental attention. Cannabis and CBD oil products are under additional scrutiny despite the fact that these claims have not been proven and do not decisively include cannabis products. In the midst of this investigation, cannabis companies must be especially careful about making unsubstantiated marketing claims regarding the use of their products and the safety precautions.
The recent lung illnesses that may be linked to the Vitamin E component in nicotine vaping oils has been garnering much public and governmental attention. Cannabis and CBD oil products are under additional scrutiny despite the fact that these claims have not been proven and do not decisively include cannabis products. In the midst of this investigation, cannabis companies must be especially careful about making unsubstantiated marketing claims regarding the use of their products and the safety precautions.
The administration sent new warning letters to undisclosed CBD product sellers regarding their usage claims. In these letters, the FTC reinforced that “it is illegal to advertise that a product can prevent, treat, or cure human disease without competent and reliable scientific evidence to support such claims.” While the letters do not mention the vaping-related illnesses, the timing of their release reminds participants in the cannabis supply chain to be especially vigilant about the safety of their products and the messaging about their health benefits. It is important to note that official studies regarding the recent lung disease has specifically named nicotine vaping products as a possible cause.
Discoveries/Innovations: CRISPR Tech Gets an Upgrade
 While CRISPR technology is generating a lot of positive buzz, scientists warn that the process isn’t as safe as all the success stories popping up would make it seem. There is good news coming from Wake Forest Institute for Regenerative Medicine (WFIRM), however. Researchers here have made strides in correcting one of the major risk factors in the CRISPR gene editing process while making it faster and more efficient.
While CRISPR technology is generating a lot of positive buzz, scientists warn that the process isn’t as safe as all the success stories popping up would make it seem. There is good news coming from Wake Forest Institute for Regenerative Medicine (WFIRM), however. Researchers here have made strides in correcting one of the major risk factors in the CRISPR gene editing process while making it faster and more efficient.
CRISPR technology is used to remove or repair certain DNA sequences to alter certain gene functions. CRISPR/Cas9 is an enzyme important in this process. It acts as the scissor that cuts the two strands of DNA at a specific location to add, remove, or repair bits of the DNA. One of the major problems in the gene editing process is that CRISPR/Cas9 is not 100% accurate and could cut unintended locations, causing unwanted and damaging outcomes.
The researchers can now package the CRISPR/Cas9 and guide RNA into a lentiviral capsid. Lentiviral vector is a widely used gene delivery vehicle that was previously safe when used for CRISPR. By combining the delivery efficiency of conventional lentiviral vectors with transient (temporary rather than long-term) Cas9 expression, the new system is safer and more efficient when it comes to targeting only the right DNA sequence.
“We have created a system that may be used for packaging various editor protein mRNA for genome editing in a ‘hit and run’ manner,” said Anthony Atala, MD, Director of WFIRM and co-lead author of the paper. “This system will not only improve safety but also avoid possible immune response to the editor proteins, which could improve in vivo gene editing efficiency which will be useful in research and clinical applications.”
Trend Setting: Cool New Tech Turns Stress into Art

The Stress Visualization Experience, unveiled in London this September, features unique technology that provides a beautiful, real-time rendering of effects of stress on the body. McCann World partnered with Cigna International Markets to combine biometric data and digital art to educate the public on how stress affects us physically and emotionally.
This effort was conceptualized after Cigna released a well-being survey that found 84% of the world population suffers from stress. Furthermore, the physical impacts of this stress can often be invisible, eventually turning into a serious side effect of chronic stress, such as hypertension, insomnia, or even diabetes. The aim of this project is to make these invisible symptoms visual.
Top-notch doctors and technologists contributed to a digital kit that gathers a user’s stress readings. The kit includes an EEG headband that measures Alpha and Beta brainwave activity, a heart rate sensor, and a stress-related skin conductance sensor. The data then flows into an algorithm that displays an artistic motion portrait that changes in shape and color to reflect the stress the user is feeling in that exact moment. The initiative hopes to help people see stress differently and be inspired to take control of it in their everyday lives.
FDA Update
Drug Approvals
The first treatment for patients with the rare lung disease SSc-ILD has been approved by the FDA. The decline in pulmonary function in adults with interstitial lung disease associated with systemic sclerosis or scleroderma can be slowed with Boehringer Ingelheim’s Ofev.
The FDA approved Kyowa Kirin’s Nourianz, an add-on therapy for adult patients with Parkinson’s disease (PD) experiencing “off” episodes, or a time when medications are not working well, causing an increase in PD symptoms.
Med Device Approvals
CVRx, Inc. was granted FDA approval for its BAROSTIM NEO System, an implantable pulse generator (IPG), a carotid sinus lead kit, and programmer system. Doctors implant the device under the skin near the collarbone and program its electrical stimulation according to the patient’s needs. The IPG delivers electrical impulses to the baroreceptors for patients with advanced heart failure.
The FDA approved the marketing of The Tether Vertebral Body Tethering System. Zimmer Biomet Spine, Inc. designed the system as a non-fusion spinal device intended to treat idiopathic scoliosis in young, developing patients. The set of screws, anchors, and cords is implanted surgically. The screw, inserted to the side of the spine, will apply tension to the cord to partially straighten the patient’s spine. After surgery, the cord continues to straighten the spine while the patient continues to grow.
Edwards Lifesciences received approval for the SAPIEN 3 Ultra Transcatheter Heart Valve System. The catheter-based artificial aortic heart valve was used to treat severe aortic stenosis in patients at intermediate and greater risk for surgical therapy. It is now expanded to include patients at low risk for surgical therapy. The SAPIEN 3 Ultra THVs can improve blood flow in patients with aortic stenosis with an implantable artificial valve that is attached to a balloon-expandable, cobalt-chromium frame for support.







