Trend Setting: Crossix Solutions Identifies New Trend
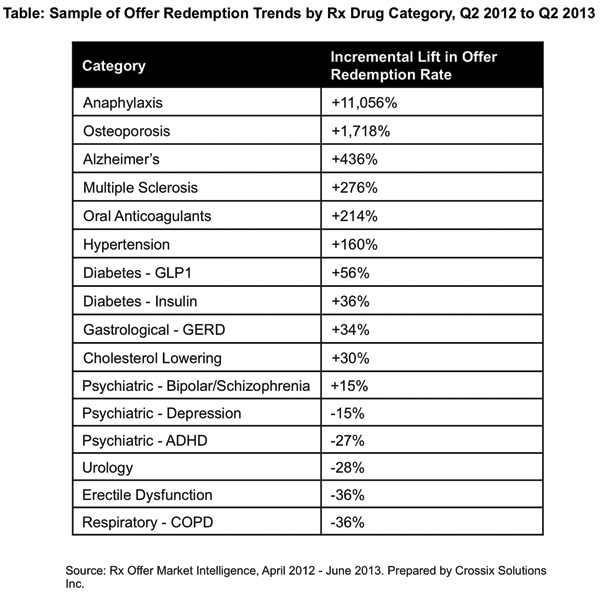
An analysis of 400 drug brands by consumer healthcare marketing analytics firm Crossix Solutions revealed that patients are taking advantage of savings offers from pharma companies. They found that patients were redeeming prescription savings offers—including co-pay offers, savings vouchers and free trial offers—nearly 20% more in Q2 2013 from Q2 2012 (See Redemption Trends By Drug Category). The company’s Rx Offer Market Intelligence (ROMI) syndicated data showed that for brands offering a savings during that time, the percentage of prescriptions filled with a savings offer increased from 6.4% to 7.6%, resulting in an 18% increase in one year. The Crossix study compared offer redemption across each major drug category, with 14 of 27 categories exhibiting increases and the other 13 categories exhibiting decreases. A full list of category trends can be viewed at www.crossix.com/romi.
Company Corner: Perthera Launches Personalized Service
Specializing in personalized therapy for patients with cancer, Perthera launched a new service, called Perthera Molecular Profiling that will deliver personalized molecular analysis of an individual’s tumor, and a detailed, and actionable treatment plan that can help prolong life. The company handles the entire process: Collecting the biopsy specimen; sending the sample for laboratory testing; generating the final report; and using an expert system, clinical knowledge base and an oncology review panel for each patient. According to the company, Perthera is the only company to offer a service that comprehensively screens for both proteins and genes in the context of a patient’s history and expert medical review. So far the company has worked with more than 50 patients since early 2013 and is already making a significant impact on how oncologists are analyzing and treating the disease.
DC Wire: Impact of Medicare Cuts on Kidney Failure Patients
In early August, with bipartisan agreement, nearly 200 Congressional members signed a letter expressing concern and caution to the nation’s top Medicare official over proposed cuts to Medicare’s End-Stage Renal Disease (ESRD) program, which covers 340,000 Americans with kidney failure. In 2014, the proposal would cut funding for dialysis by 12%, or $30 dollars on each $246 payment that Medicare would have paid per treatment. Congress was tipped off by countless professional kidney organizations, the healthcare and patient communities and providers who expressed the negative consequences the proposed cuts would have on staffing, access and quality of care. Kidney care and community leaders said that if the proposed rule becomes final, Medicare would fail to cover the cost for each beneficiary’s dialysis session. Leaders are urging the Centers for Medicare and Medicaid Services to reverse course before the rule is finalized following the 60-day comment period, which ended Sept. 1.
Discoveries/Innovations: Discovery of New Opioid
Opioids, such as morphine, are standard treatment for people with chronic pain, but at higher dosages they can cause debilitating side effects such as constipation, nausea, drowsiness and dizziness. However, researchers from University of Maryland’s School of Pharmacy developed a new opioid compound, known as UMB 425, that is as strong as morphine, but has diminished tolerance over time with no obvious toxic effects. The researchers have undergone several preliminary in-vitro and in-vivo studies, but if future research and clinical trials are successful, UMB 425 could have a significant impact in pain management and palliative care.
Patient Pages: GfK Study Results on Personalized Medicine
Marketing research company, GfK, recently conducted a study that analyzed the population’s knowledge of the term “personalized medicine” (PM), their perceptions of it, its impact on healthcare and the desire to learn more about it. The study surveyed 602 individuals online (both male and female), ages 30 and above, of varying educational backgrounds and income ranges across the country. The results: The general population has low familiarity with PM—73% of total respondents have not heard the term before, and 41% did not know what the term meant. The study also showed that:
- Most respondents have a vague or false understanding of PM. Only 41% of those familiar with PM accurately describe it as “medicine based on genome/genetic make-up.”
- 67% of respondents were more interested in genetic testing if they had been diagnosed with life-threatening cancer.
- Those interested in genetic testing were more educated, had more health conditions, a work-sponsored health plan and saw the value of PM.
- Respondents were interested in receiving info concerning costs, their personal genetic profile and other personal health info.
Sales Sector: Interactions with Tablet-Carrying Sales Reps
According to the spring 2013 AccessMonitor report from global consulting firm ZS Associates, 65% of oncologists in the U.S. placed moderate to severe restrictions on visits from pharmaceutical sales reps. By comparison, only 17% of oncologists restricted access to reps in 2008. Oncology is the most restrictive of the 20 common medical specialties measured in the report.
The report also revealed that sales reps who offer one or two products see oncologists an average of seven times per year. Sales reps carrying three or more products are able to visit physicians an average of 10 times per year. On average, a rep carrying three or more products is twice as likely (26%) to have access to even the most restrictive oncologists (versus 13% for reps carrying one or two products).
The report concluded that while a company may not be able to launch multiple products per year, the key to access for pharma is to think differently about how their reps can deliver unique, tailored and relevant offerings to oncologists. For a summary of the report, go to: bit.ly/ZS-AccessMonitor2013.
FDA Update
FDA Releases Final Guidance on Medical Apps
The FDA issued final guidance for developers of medical apps for smartphones and tablets and laid out what their oversight of mobile medical apps will be. According to the guidance, the FDA will focus on mobile medical apps that are intended to be used as an accessory to a regulated medical device and/or transform a mobile platform into a regulated medical device. Mobile medical apps that undergo FDA review will be assessed using the same regulatory standards and risk-based approach that applies to traditional medical devices.
New Approvals
Janssen’s Stelara (ustekinumab) got approval for the treatment of adults with active psoriatic arthritis. Stelara is a fully human interleukin-12 and interleukin-23 antagonist used for treating adults with moderate to severe plaque psoriasis.
Teva gained FDA approval for the first generic version of Xeloda (capecitabine), an oral chemotherapy pill used to treat metastatic colorectal cancer and metastatic breast cancer. The drug is approved to be marketed in 150 and 500 milligram strengths.
Perrigo received final approval for nitroglycerin lingual spray, 400 mcg/spray, the generic equivalent to Nitrolingual Pumpspray. The product is indicated for the acute relief of an attack or prophylaxis of angina pectoris due to coronary artery disease. Perrigo was awarded 180 days of generic drug exclusivity. The company also got the go-ahead to market bubble gum flavored cetirizine hydrochloride oral solution USP, one mg/mL, the store brand equivalent to Children’s Zyrtec Allergy Syrup for children ages two and above who experience indoor and outdoor allergies. Perrigo expects to begin shipments of the product during the upcoming cough/cold/flu season.