TeleMed Texts: Medical Marijuana Delivery Service Starts—On Demand
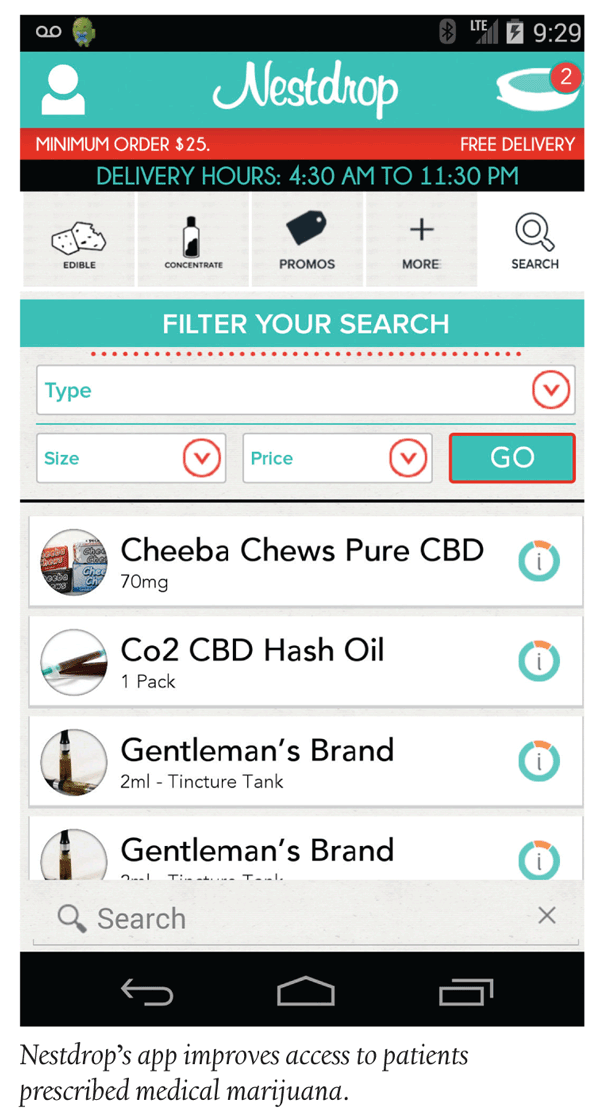
A first in-app launch by Nestdrop is an on demand medical marijuana delivery service for iPhones and Androids. Launched in June 2014, it is touted as offering a safer, more efficient way to order medical marijuana.
The company worked with local doctors to categorize the available medicine based on the relief it provides, and now medical marijuana patients can access Nestdrops’s medical marijuana service by uploading a photo of a doctor’s recommendation and a Medical Marijuana Identification Card. Within minutes of being approved by a local dispensary, patients will have full access to the same medicine that they could also receive in-person.
“We saw how this type of platform could be used to bring difficult-to-obtain products to people who really need them,” said Nestdrop Co-founder Michael Pycher. “We began talking to patients and found a genuine need for improved access to this medicine.”
Patient Pages: PatientsLikeMe and CTCA Support Cancer Survivors
PatientsLikeMe and Cancer Treatment Centers of America (CTCA) at Eastern Regional Medical Center (Eastern) will be working together to provide a fully integrated approach to cancer treatment. The collaborated effort will provide an online network of support for patients transitioning from treatment to survivorship with direct access to online resources and information. The addition will not only provide support and services for patients, but will also contribute data for future cancer research.
“Our members share information about how they’re managing their condition and actively contribute insights and data to help others and advance research,” said PatientsLikeMe Executive of Marketing, Michael Evers, in a statement. “We’re excited to welcome the CTCA community to PatientsLikeMe. Together, we’ll give everyone the support, tools and services they need to be fully empowered on their journey with cancer.”
Trend Setting: Five Disruptors Could Cost Med Device Industry
Five major disruptors will negatively impact the economics of the medical device industry, according to an A.T. Kearney report, Medical Devices: Equipped for the Future? The study provides recommendations for medical device industry executives to address this disruptive change. The five disruptive forces shaping the medical device industry are:
1. Power shift to payers and providers: Payers and providers are evaluating medical devices based on safety and procedural efficacy, as well as cost and value.
2. Heightened regulatory scrutiny: In recent years, companies have seen more action from the FDA. FDA audits have increased by 40% in the past 12 months and the number of warning letters rose by 24% over the past two years.
3. Unclear sources of innovation: Because of regulatory and reimbursement issues, medical device companies are focusing their R&D efforts on improving already approved devices rather than developing truly innovative new products.
4. New healthcare delivery models: Care is shifting out of hospitals and into the community.
5. Need to serve lower socioeconomic classes: Medical device companies seeking growth will need to target less affluent segments that can offer significant absolute profit potential with the right solutions.
Therapeutic Talk: Stethoscope Adaptor Could Cut Cardiac Costs
Eko Devices developed a small adapter attachment for stethoscopes that captures high-quality recordings of the heart. These sounds are transferred through Bluetooth where they automatically go to the cloud, via a connected smartphone or tablet. The acoustic pattern is compared to a library of over 30,000 heart sounds to identify the most likely cause of an abnormality. By using this connected mobile device, any physician or specialist can re-listen to the heart sounds and also review the acoustic signal. The Eko Device creates an opportunity to help primary care physicians identify benign heart sounds, detection of serious heart abnormalities without unnecessary imaging or specialty evaluation.
With this device, Eko Devices makes its first step toward harnessing the power of smartphones and cloud computing to help physicians make data-driven decisions to improve patient outcomes and reduce healthcare costs. The device has yet to receive FDA clearance, though that is reportedly expected in the next year.
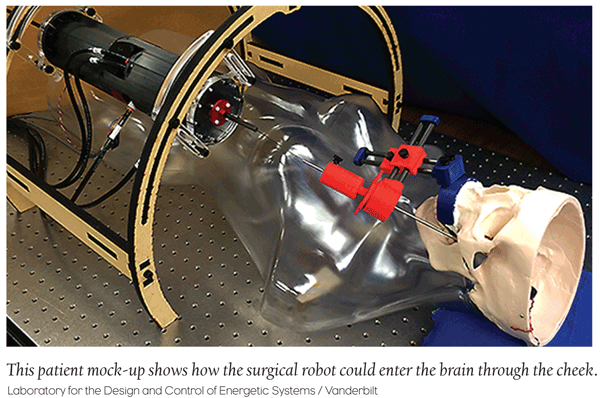
Medical Device: Surgical Robots Perform Brain Surgery
Engineers at Vanderbilt University developed a surgical robot capable of performing brain surgery to treat epilepsy. The new robot eliminates the need to perform invasive procedure removal of the hippocampus, the portion of the brain responsible for seizures. The robot can access the hippocampus more directly through the cheek. During lab testing, the accuracy of the robot is better than 1.18 millimeters, which is regarded as sufficient for this type of surgical procedure.
Joseph Neimat, Associate Professor of Neurological Surgery said in a statement, “The systems we have now that let us introduce probes into the brain—they deal with straight lines and are only manually guided. To have a system with a curved needle and unlimited access would make surgeries minimally invasive. We could do a dramatic surgery with nothing more than a needle stick to the cheek.”
FDA Update
FDA Regulations Increase
A 15% increase in the number of FDA regulatory requirements took place between 2002 and 2012. According to George Mason University’s Mercatus Center data, which was analyzed by the Regulatory Review, FDA regulatory officials needed to know only 16,329 requirements in 2000. In 2012, the number of regulations increased to 18,777.
Drug Approvals
AbbVie’s Humira gained FDA approval for an extension to the drug’s indication for moderately to severely active polyarticular juvenile idiopathic arthritis (JIA) to include reducing signs and symptoms in patients ages two and older.
Gilead also gained approval for its hepatitis C combination drug, Harvoni (ledipasvir/sofosbuvir), to treat adults with chronic hepatitis C virus (HCV) genotype 1 infection. The drug was first approved as Sovaldi in December of 2013.
Priority Review
Genentech was granted Priority Review of Lucentis (ranibizumab injection) for the treatment of diabetic retinopathy. The FDA confirmed action date is February 6, 2015.
Breakthrough Therapy Designation
Merck received a Breakthrough Therapy Designation for Keytruda (pembrolizumab), an anti-PD-1 therapy, for the treatment of epidermal growth factor receptor (EGFR) mutation-negative, and anaplastic lymphoma kinase (ALK) rearrangement-negative non-small cell lung cancer (NSCLC) in patients whose disease has progressed on, or following platinum-based chemotherapy.
Based on interim efficacy and safety results from an ongoing Phase I/II study, Clovis Oncology received Breakthrough Therapy Designation for investigational agent CO-1686 as a monotherapy for the treatment of second-line EGFR mutant NSCLC in patients with the T790M mutation.