Doctor Docs: New York Requires Docs to Go Digital
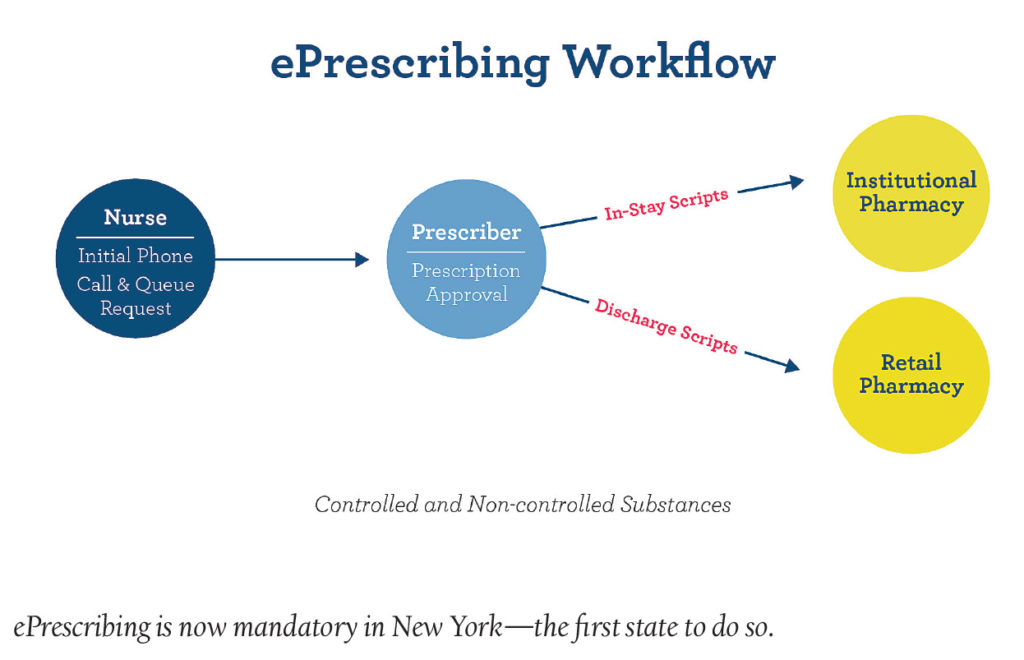 New York is the first state to require all prescriptions to be submitted electronically to pharmacies, which is having ramifications on both doctors and patients alike. Doctors must submit an ePrescription to a pharmacy of the patient’s choosing, but problems occur when the prescription needs to be reissued. If the drug cost proves too high, patients will have a much harder time having their prescriptions sent to another pharmacy. This difficulty is also leading doctors to prescribe more general, popular drugs that are more likely to be in stock rather than more specialized drugs that are more likely to be helpful to the patients. The change in systems is meant to cut down on the massive opioid abuse problem throughout the U.S.
New York is the first state to require all prescriptions to be submitted electronically to pharmacies, which is having ramifications on both doctors and patients alike. Doctors must submit an ePrescription to a pharmacy of the patient’s choosing, but problems occur when the prescription needs to be reissued. If the drug cost proves too high, patients will have a much harder time having their prescriptions sent to another pharmacy. This difficulty is also leading doctors to prescribe more general, popular drugs that are more likely to be in stock rather than more specialized drugs that are more likely to be helpful to the patients. The change in systems is meant to cut down on the massive opioid abuse problem throughout the U.S.
Medical Devices: Skin Patches for Diabetes
Scientists are developing a skin patch that will not only detect glucose levels of diabetes patients, but administer medication as needed. Scientist Dae-Hyeong Kim from Seoul National University led a team of researchers at MC10 to develop the prototype that will allow diabetes patients to avoid pricking their fingers and injecting drugs every day. The patch monitors glucose levels in sweat and then uses these levels to determine when to heat micro-needles that administer drugs.
The MC10 device would be the first minimally invasive tool for assessing glucose levels and treating them. Past models were pulled from the market, as they caused sores and discomfort. In addition, the MC10 device will store drug administration information and transfer it to an app that can be easily accessed on a smartphone.
TeleMed Texts: The Newest Calorie Tracker—Your Necklace
 Wenyao Xu has recorded people chewing hundreds of different foods, building the sound library of AutoDietary, a next generation wearable that will track caloric intake by listening to you chew. The small necklace records the sound of the wearer chewing and swallowing and sends it to a smartphone app that recognizes the food. So far, Xu’s studies at the University of Buffalo have proven that AutoDietary can accurately predict the food 85% of the time.
Wenyao Xu has recorded people chewing hundreds of different foods, building the sound library of AutoDietary, a next generation wearable that will track caloric intake by listening to you chew. The small necklace records the sound of the wearer chewing and swallowing and sends it to a smartphone app that recognizes the food. So far, Xu’s studies at the University of Buffalo have proven that AutoDietary can accurately predict the food 85% of the time.
“There is no shortage of wearable devices that tell us how many calories we burn, but creating a device that reliably measures caloric intake isn’t so easy,” says Xu, PhD, Assistant Professor of Computer Science in UB’s School of Engineering and Applied Sciences. Xu is working on the limitations that audio records impose, such as it not being able to tell the difference between regular or frosted corn flakes, which does make a caloric difference. With time, AutoDietary can go beyond being a weight loss instrument to be a huge help to those with diet-related diseases, such as diabetes.
Therapeutic Talk: Can a Sponge Treat Spinal Tumors?
Researchers at the Mayo Clinic, Minnesota, are working on a treatment for metastatic spinal tumors using the same concept as the rapidly growing sponges used in everyday life. Currently, treating these tumors either requires opening the entire chest cavity to fit metal cages or bone grafts to support the spine, or making a small cut in the back, which doesn’t allow enough room for the supports needed for many surgical treatments. Researchers Lichun Lu, PhD and Xifeng Liu, PhD have developed a tube of “sponge” material that can be fitted with the posterior surgical entrance, which then expands to the correct size and at the correct rate to fill the space necessary once it is hydrated by the body.
Discoveries/Innovations: Steering Bacteria-Powered Microbots in the Body
For a few years, using microbots to deliver medication on the cellular level has been an active area of research, helping to treat cancer and other diseases. However, the problem is often that the direction of the microbots, propelled by the waving flagella of attached bacteria, cannot be controlled once in the body. MinJun Kim, PhD and professor in the College of Engineering at Drexel University, is hoping to control the path of microbots with electric fields. “We have shown that we can manually direct the robots or give them a set of coordinates to get it from point A to point B, but our goal in this research is to enable the microbots to navigate a course with random impediments blocking its way,” Kim said. “This requires a level of automation that has not previously been achieved in hybrid microrobotics research.”
Kim’s finding are allowing him and his team to build an algorithm that will tell the bacteria-steered robots how to judge which obstacles to avoid and which to ignore. These basics are important to master before programming the robots to perform more complex methods of treatment, like delivering medication.
FDA Update
FDA Approvals
Titan Pharmaceuticals and Braeburn Pharmaceuticals recently received FDA approval for Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is a subdermal implant that delivers a continuous low-level dose of the drug for up to six months in patients who are clinically stable and are already using low to moderate doses of others types of buprenorphine.
Biogen and AbbVie received FDA approval for Zinbryta (daclizumab) for relapsing forms of multiple sclerosis. Zinbryta is a once-monthly, self-administered, subcutaneous treatment that is indicated for use in patients who have inadequate responses to two or more MS therapies.
Teva’s Cinqair (reslizumab), recently received FDA approval for combination use with medicines used as maintenance treatment of severe asthma in patients 18 and older. It is indicated for use in patients with continuing severe asthma exacerbations despite the use of other asthma treatments.
Intercept Pharmaceuticals was recently awarded FDA accelerated approval for Ocaliva (obeticholic acid) for the treatment of adults with primary biliary cholangitis (PBC). It is a farnesoid X receptor agonist for use in combination with ursodeoxycholic acid in patients with an inadequate response to ursodeoxycholic acid or as a single therapy for those who cannot tolerate ursodeoxycholic acid.
Medical Device
Parker Hannifin received FDA approval for the Indego exoskeleton for use by paraplegics in both personal and clinical settings. It is used to increase mobility and for rehabilitation in patients with lower limb injuries.






