Med Device: Capsule Endoscopy Offers Upgrades, Real-Time Viewing
A new Endocapsule 10 System developed by Olympus is a state-of-the-art technology for small bowel capsule endoscopy procedures that features advancements in visualization, efficiency and user features, according to the company. The system boasts advanced lens and sensor technologies, as well as a capsule with a 160° field of view, as opposed to the 145° field of view provided by other endoscopy technologies. The system also offers an extended battery life of 12 hours, dramatically increasing the number of images taken during the procedure. New and improved software allows the system to automatically detect images (which can be viewed in real time) that require further analysis and to indicate the location of each abnormality, according to the company.
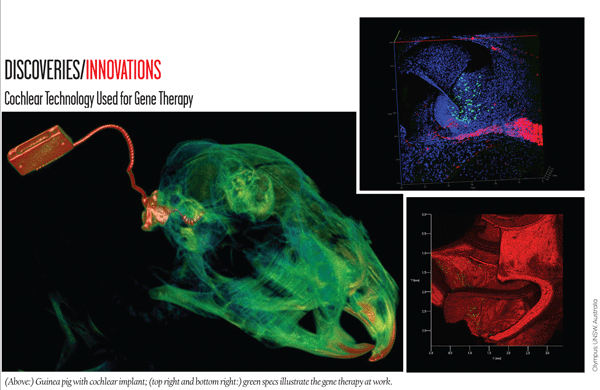
Discoveries/Innovations: Cochlear Technology Used for Gene Therapy
Research from the University of New South Wales Australia shows that electrical impulses from a cochlear implant can now be used to re-grow auditory nerves through localized gene therapy. According to the research paper, while users of cochlear implants can generally understand speech, this new therapy allows for the comprehension of a broader range of sound—including music.
The research focuses on regenerating damaged nerves in the auditory portion of the ear and improving their connections with nearby electrodes. While previous research concentrated on delivering neurotrophins, a type of protein necessary for the healthy development of neurons, this technique uses the implant’s electrical impulses to deliver the DNA and cause the cells near the electrodes to produce neurotrophins themselves. This new method of delivery of localized gene therapy could also potentially be used to treat a host of neurological and psychiatric disorders.
Sales Sector: Physician Engagement Requires Country-Specific Approach
A recent Manhattan Research study from Decision Resources group found that it is critical for marketers to tailor their approach to foreign markets according to their physicians’ technological maturity and channel preferences. According to Manhattan Research’s Taking the Pulse Global 2014 study, these factors vary widely from country to country and market to market and affect marketers’ opportunities for physician engagement.
The study found that opportunities in the Australian, Brazilian and Chinese markets include:
EHRs: 72% of physicians in Australia have adopted this platform and physicians spend a great deal of time using EHRs for a number of clinical activities, so marketers need to create a presence across EHR platforms for this audience.
Tablet reps: This year, three out of four Brazilian physicians have seen a tablet rep. Despite this high number, the study also found that improvement is needed in the areas of content and user experience to bolster physician satisfaction.
Smartphones: Four out of five physicians in China use a smartphone for professional purposes, requiring marketers to create tailored content to reach these healthcare professionals.
“A one-size-fits-all digital global strategy will not suffice for optimal engagement with physicians today,” notes Director of Physician Research James Avallone. Global markets have become so differentiated, he adds, that marketers can no longer employ a universal marketing approach.
DC Dispatch: Public Citizen Sues FDA To Improve Warnings for PPIs
Watchdog group Public Citizen filed a lawsuit on April 30, 2014 alleging that the FDA illegally withheld action regarding inadequate warnings for proton pump inhibitors (PPIs), drugs commonly prescribed to treat acid reflux. Studies have shown that PPIs can increase the risk for bone fractures, cardiac arrhythmias and certain types of infection. The lawsuit states that the warning labels that detail these risks are not prominent enough and that another potential risk is not mentioned at all.
According to a 2009 study, patients who take PPIs frequently may also be at risk for dependence on the drugs. While many take this drug chronically, it was originally intended for short-term intervention. The lawsuit seeks a court order to compel the FDA to act within 30 days in service of making the existing warning labels more prominent, as well as adding the risks associated with frequent or chronic usage.
FDA Update
FDA Actions
The U.S. Food and Drug Administration took action to stop online pharmacies that advertised and sold prescription drugs that had not been approved by the proper authorities. They coordinated with U.S. Customs in order to examine packages at international mail facilities for illegal and potentially dangerous drugs. This resulted in the seizure of 19,618 packages from all over the world.
FDA Approvals
The FDA approved Zontivity, Merck Sharp & Dohme Corp.’s prescription tablets intended to treat cardiovascular diseases such as heart attack, stroke and cardiovascular death—the first in a new class of drugs that decreases the formation of blood clots. Durata Therapeutics’ Entyvio, a drug meant to treat inflammatory bowel diseases also received approval and is now an option for patients who have become intolerant to conventional therapies. The FDA also approved an expanded label for Vectibix from Amgen, Inc. that authorizes the drug for the treatment of metastatic colorectal cancer.
Fast-Tract Designation
BioAlliance Pharma announced that Livatag, which treats hepatocellular carcinoma (primary liver cancer) has received FDA Fast Track designation. Livatag is a chemotherapy drug that uses a nano particulate formation to overcome the resistance mechanisms of cancer cells. In the U.S., it is the second-line treatment of primary liver cancer, to be used after the drug sorafenib.