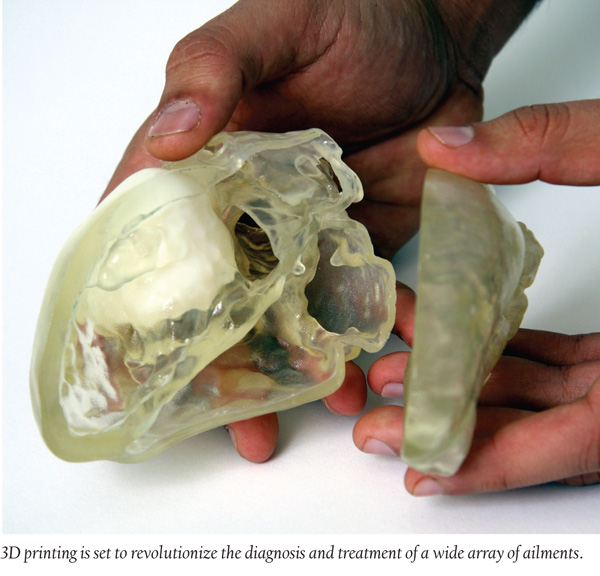
Trend Setting: 3D Printing: The Future of Personalized Healthcare?
Materialise, a 3D printing service, announced a new service offering, the Materialise Hospital Solution, which will give clinicians the ability to translate patient-specific data into an accurate and tangible model that will make it easier for clinicians to address the needs of each individual patient, according to the company.
3D software and models can be used to explain a patient’s condition to families, assist in diagnosing complex pathologies and even support testing a procedure on the surgical team and bench before going to the bedside. It is also a powerful resource, the company adds, to assist in the diagnosis of patients and provide additional evidence for determining treatment plans.
“It’s hard to imagine entering an operating room for another complex case without the aid of a 3D printed model. It’s definitely going to be standard of care in the future,” Dr. Emile Bacha, a congenital heart surgeon and Director of Congenital and Pediatric Cardiac Surgery at NY-Presbyterian/Morgan Stanley Children’s Hospital, said in a statement.
Discoveries/Innovations: A New Way To Turn Genes On
Researchers at MIT made a significant breakthrough with the new application of the CRISPR/Cas9 gene-editing system. They are using it to turn on any gene in a living cell. The CRISPR system was originally designed to delete specific genes, but can now be used to replace and delete genes. With a revamped system, a dozen genes that had proven difficult or impossible to turn on using the previous generation of Cas9 activators can be activated.
This new application of the CRISPR/Cas9 gene-editing system enables rapid functional screens of the entire genome, which allows scientists to identify individual genes involved in particular diseases, according to Feng Zhang, the W.M. Keck Career Development Professor in Biomedical Engineering in MIT’s Departments of Brain and Cognitive Sciences and Biological Engineering.
Scientists have tried to do this before using proteins that are individually engineered to target DNA at specific sites. However, their attempts were unsuccessful in consistently turning on gene transcription until now.
TeleMed Texts: Rapidly Accessing Cardiac Device Data
A technology platform for managing data from cardiac devices, the new Geneva Healthcare Suite, will soon be operating in all Sharp Healthcare hospital emergency departments nationwide—and perhaps others soon.
According to the company, the platform allows emergency department operators to efficiently access a patient’s cardiac device data, use that information at the point of care and immediately make this information accessible to the most qualified clinician wherever they are.
“This tool allows our physicians and nurses to assess and diagnose patients with implanted cardiac devices much more rapidly and efficiently, and it enables us to identify potential problems before they become serious,” Pablo Velez, CEO of Sharp Chula Vista Medical Center, said in a statement. “The result has been improved care for our patients—which is always the number one priority.”
Doctor Docs: DocNews Now Reaches 40% of U.S. Physicians
In a recent survey, physician news network, Doximity, revealed that nearly 75% of physicians make changes to the procedures of their medical practices on a quarterly or monthly basis, based on the new information they read in medical literature. But keeping up to date on valuable medical literature can be challenging for physicians as there are more than 7,000 published medical journals to sort through monthly.
Doximity claims that its professional network, DocNews, now reaches 40% of U.S. physicians and helps keep them up to speed. The network allows physician members to automatically receive news suited to their professional interests. Washington, D.C.-based surgeon, Dr. Hassan Tetteh, said in a statement, “Having a secure dynamic platform like Doximity to share knowledge and communicate with my medical colleagues is priceless.”
Therapeutic Talk: New Seven-day Patch for Parkinson’s Disease
Teikoku Pharma USA announced positive results of a bioavailability study examining the delivery of a seven-day patch for Parkinson’s disease. The study confirmed that one patch of rasagiline TPU-002RA achieved a higher systemic exposure when applied in seven days than the Azilect rasagliline tablets.
Paul Mori, Executive Vice President and COO said in a statement, “We are pleased to have demonstrated the ability of our unique transdermal drug delivery technology to provide systemic drug exposure equivalent or greater than the oral product at its therapeutically effective dose.”
This study was part of the FDA’s recommended program for a 505(b)(2) New Drug Application (NDA) for TPU-002RA and will be a critical component of this product’s non-disclosure agreement. The results showed that the patch produced more consistent blood levels of rasagliline throughout the seven-day period, without any significant peaks—as seen with the oral product. The patch, according to Mori, offers the potential for improved medication adherence and reduction of the caregiver workload.
FDA Update
Possible New Legislation
A bill introduced by Senators Michael Bennet (D-CO) and Orrin Hatch (R-UT) will be worth keeping an eye on in the New Year. The Promise for Antibiotics and Therapeutics for Health (PATH) Act would establish a new pathway for antibacterial drugs treating a serious or life-threatening disease that affects a specific, limited population. This could allow new treatments to get to affected populations faster.
New Approvals
Gilead’s Sovaldi and Harvoni now have some competition. AbbVie’s Viekira Pak was approved to treat patients with chronic hepatitis C virus (HCV) genotype 1 infection, including those with cirrhosis, and Express Scripts has already chosen Viekira Pak as its exclusive option for HCV patients. AstraZeneca’s Lynparza was granted accelerated approval as a treatment for women with advanced ovarian cancer associated with defective BRCA genes, as detected by an FDA-approved test. Cubist’s Zerbaxa was approved to treat adults with complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI).
Electronic Labeling
A proposed new rule, Electronic Distribution of Prescribing Information for Human Prescription Drugs, Including Biological Products, would require a product’s container label and outside package to provide a link directing HCPs to the electronic version of the prescribing information (http://1.usa.gov/1xbrTZg). This only applies to professional labeling and not patient labeling, as the intended purpose is for HCPs to have the most current prescribing information when they are making clinical decisions. This also requires manufacturers to update the product labeling at FDA’s labels.fda.gov website anytime a change is made.