Medical Device: Verily Launches Prescription Only Smart Watch

Verily, Alphabet’s division formerly known as Google Life Sciences, introduces their new, FDA-approved and ECG-equipped Study Watch. The device was originally intended for clinical studies, specifically Project Baseline—a 2017 venture by Google, Stanford Medicine, and Duke University that tracked cardiovascular metrics of 10,000 volunteers for four years. Now, a new version of Study Watch is available via prescription for adults with heart conditions. The device is designed to measure various physiological and contextual data through sensors and unobtrusive biosensing that blends into daily life to ensure long-term wear and better data collection. Data from Study Watch are uploaded to Verily Study Hub, and are processed in the cloud using backend algorithms and machine learning tools.
Study Watch has been cleared to record, store, transfer, and display single-channel ECG rhythms to be used for individual care between patients and HCPs, as well as population-based research. Verily remains focused on using Study Watch for cardiovascular health since heart disease remains a number one killer in the U.S. despite its preventability.
Trend Setting: Clinician-controlled Robot Completes First Heart Procedure
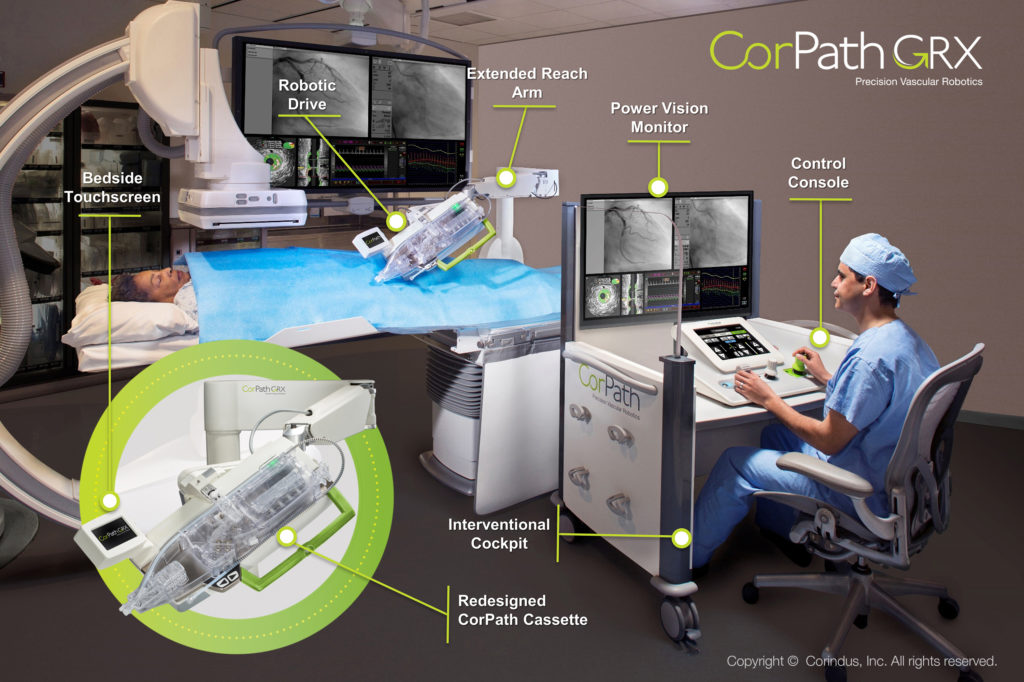
Apex Heart Institute of India performed the world’s first percutaneous coronary intervention (PCI) conducted with a vascular robot controlled by a physician located 20 miles away. Five patients at Apex in Ahmedabad, Gujarat, underwent the PCI procedure while Dr. Tejas Patel, Chairman and Chief Interventional Cardiologist of the Apex Heart Institute, controlled Corindus Vascular Robotics’ CorPath technology from inside the Swaminarayan Akshardham temple located in Gandhinagar. Dr. Patel states that “The application of telerobotics in India has the potential to impact a significant number of lives by providing access to care that may not otherwise have been possible.”
The robotic device allows for inhumanly precise placement of stents, balloons, and devices that unblock patients’ arteries and stay in place to prevent clotting and other arterial complications. Cardiovascular disease is the number one killer in the world, resulting in 18 million deaths each year. Geographic barriers, socioeconomics, and a shrinking number of skilled specialists contribute to this number since patients often cannot access timely, specialized cardiovascular care in emergency cases such as heart attack and stroke. Corindus developed the remote telerobotic interventional platform to deliver highly specialized and timely cardiovascular care to underserved patient populations with physical barriers to treatment. Following the successful FIH telerobotic coronary stenting cases performed in India, the company plans to begin commercial product development for use of the CorPath System in remote interventions.
Discoveries/Innovations: AI Detects Genetic Disorders from Face Photo
A new AI technology dubbed DeepGestalt is able to identify several genetic disorders by scanning a photograph of a patient’s face. FDNA, an artificial intelligence and precision medicine company responsible for the development, claims that the AI program is much better than physicians at identifying diseases such as Angelman Syndrome or Noonan Syndrome. Having been trained with 17,000 images of patients with nearly 200 genetic disorders, DeepGestalt was able to correctly diagnose Noonan syndrome 64% of the time as opposed to clinicians (20%) using facial characteristics the researchers and doctors were not able to pinpoint.
“DeepGestalt demonstrates how one can successfully apply state-of-the-art algorithms, such as deep learning, to a challenging field where the available data is small, unbalanced in terms of available patients per condition, and where the need to support a large amount of conditions is great,” said Yaron Gurovich, Chief Technology Officer at FDNA. While Gurovich is convinced that this technology is a revolutionary step towards identifying genetic disorders in a clinical setting, he and the other authors of the research warn that this could lead to payers and employers potentially analyzing easily accessible facial images and discriminating against individuals who have pre-existing conditions or developing medical complications. In any case, DeepGestalt is a shining example of how AI technology can be used to change lives for the better.
TeleMed Texts: Upwell Health Launches Hub for Chronic Condition Patients
Taking another step in its mission to provide services and support for people living with chronic conditions, Upwell Health launched UpwellBeing.com, a website with educational videos and content to help people living with conditions related to heart health, mental health, diabetes, and dermatological health take proactive steps towards healthier living. UpwellBeing will publish daily news, videos, reports, research, and infographics to help people live healthier, more empowered lives by better understanding their conditions and the resources available to them.
Upwell, dedicated to offering numerous services to ensure members receive the medications they need, with the choice, flexibility, and transparency they deserve, appointed new Chief Strategy Officer Bridget Quinlan to drive strategic direction and oversee UpwellBeing. “Instead of looking at patients as facilitators to revenue or defining our members by a single condition, we view them as the complex people they truly are,” said Quinlan in a statement. “I look forward to helping the company take a holistic approach to understanding our members’ needs and empowering them with choice in an industry where choice has been stripped away.”
Brand Beat: First Cannabis-based Drug Hits the Market
GW Pharmaceuticals developed the first plant-based cannabis drug, Epidiolex, to reach U.S. markets at the recent J.P. Morgan Healthcare Conference. The FDA-approved drug is a prescription pharmaceutical formulation of highly purified, plant-derived cannabidiol (CBD), a cannabinoid lacking the high associated with marijuana. The drug is the first in a new category of anti-epileptic drugs meant for difficult-to-treat conditions such as Dravet Syndrome and Lennox-Gastaut syndrome (LGS). So far more than 2,000 patients have been treated with the drug, including an 8-year-old Dravet Syndrome patient, Grace, whose life has improved drastically with the drug.
While Epidiolex has launched in the U.S. for $32,000, CEO Justin Gover has announced they’re moving forward with indications for treatment of tuberous sclerosis complex (TSC) and Rett Syndrome, and expect a European launch in the near future. The novel cannabinoid has a seemingly strong place in the epilepsy market since approved LGS drugs exist, but no new drugs have been developed in eight years and many of these patients must switch drugs over time.
FDA Update
Drug Approvals
The FDA approved Exelixis Inc.’s Cabometyx, an oral drug intended to treat patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
Alexion Pharmaceuticals received FDA approval of Ultomiris for adult patients with paroxysmal nocturnal hemoglobinuria (PNH), a rare, life-threatening disease of the blood usually affecting patients 35-40 years old, that destroys red blood cells, causes blood clots, impairs bone marrow function, and often causes death within 10 years. Ultomiris is the first and only long-acting C5 complement inhibitor administered every eight weeks via IV.
The FDA approved Torrent Pharmaceuticals’ generic for schizophrenia medication Latuda. The lurasidone hydrochloride tablets treat schizophrenia and depressive episodes associated with Bipolar I Disorder (bipolar depression) in adults.
Elzonris, a CD123-directed cytotoxin, received FDA approval to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and in pediatric patients 2 years and older. Stemline Therapeutics developed the IV drug to treat the acute myeloid leukemia, which progresses through bone marrow and blood.
The FDA approved the first generic version of Sabril (vigabatrin) tablets as therapy for patients 10 years and older who haven’t responded adequately to certain alternative treatments. Teva’s 500mg vigabatrin tablets treat complex partial seizures, also known as focal seizures.
Med Device Update
Teva’s ProAir digihaler is the first FDA approved digital inhaler with built-in sensors to detect each time it is used to administer treatment and the inspiratory airflow during each inhalation. The data is uploaded to an accompanying smartphone app via Bluetooth wireless connectivity to be used by the patients and their HCPs for better asthma management.
The Zenith Dissection Endovascular System is a Cook Medical stent approved to treat patients with a tear in the lining of the large artery in their chest (descending thoracic aorta), which allows blood to flow between the layers of the aortic wall.






