Trend Setting: A Robotic Arm Could Improve Life for Visually Impaired
Researchers are developing a robotic device for those who are blind or have serious visual impairment to wear on their hands to help them sense and navigate their surroundings. The device’s high-resolution cameras will first notify the wearer of the location of on object. As the hand reaches towards the object, proximity and touch sensors will “describe” the shape of the object to the hand, so it can be used safely and easily by the wearer. Dr. Yantao Shen, leader of the project at University of Nevada, Reno’s College of Engineering, says, “Not only will this device help blind and visually impaired people, the methods and technology we develop will have great potential in advancing small and wearable robot autonomy with many potential applications in space exploration, military surveillance, law enforcement and search and rescue.”
Med Device: New Blood Vessels Via 3D Printing?
3D printing is about to revolutionize the way we look at the human body. Researchers at Lawrence Livermore National Laboratory are replicating the intricate human cardiovascular system using advanced 3D printing technologies. The goal is to have real blood vessels connect with artificial implanted tubes so nutrients can be delivered to the living cells, bypassing any injury or abnormality. The bio-printed cells would be placed in the human environment that directs them to reproduce. Lead investigator at the lab, Monica Moya, says, “We’re leveraging the body’s ability for self-directed growth and you end up with something that is more true to physiology. We can put the cells in an environment where they know, ‘I need to build blood vessels.’ With this technology we guide and orchestrate the biology.” With more research, 3D printing technologies will be able to produce more high-resolution products, delivering new research opportunities.
TeleMed Texts: Medical Marijuana is Only a Video Call Away
Medical marijuana patients will soon be able to connect to their providers via video call with the EazeMD app. Eaze, a San-Francisco based company, will require all patients to fill out the same forms required by California directly on the app before speaking with a licensed physician between 11:00 a.m. to 7:00 p.m, seven days a week. At $30 for a consultation, EazeMD may be cheaper than visiting a clinic. The patient can immediately order marijuana from the app once approved. The ability to legally order medical marijuana on the Internet in minutes is stirring controversy, but Eaze has already made its app available to currently approved medical marijuana patients.
Therapeutic Talk: “Bubbles” Could Treat Liver Cancer
Researchers bypassed conventional cancer research methods and repurposed a topical medicine, bexarotene, for experimental liver cancer treatments. Self-assembling nanobubbles filled with the medicine are delivered directly to liver tumors where ultrasound ablation is then used to pop the bubbles and release the agent. As liver cancer usually requires extensive surgery, this treatment option would be able to deliver effective medicine straight to the source with minimally invasive techniques. Dipanjan Pan, Assistant Professor of Bioengineering at the University of Illinois at Urbana-Champaign, claims the process is also safe. “The probability of its undesired systemic release is minimal due to this highly selective activation mechanism, which helps to spare the healthy cells,” he explains. Researchers hope to soon test the method in humans.
Doctor Docs: Veeva’s Cloud-Based Solution Will Empower KOLs
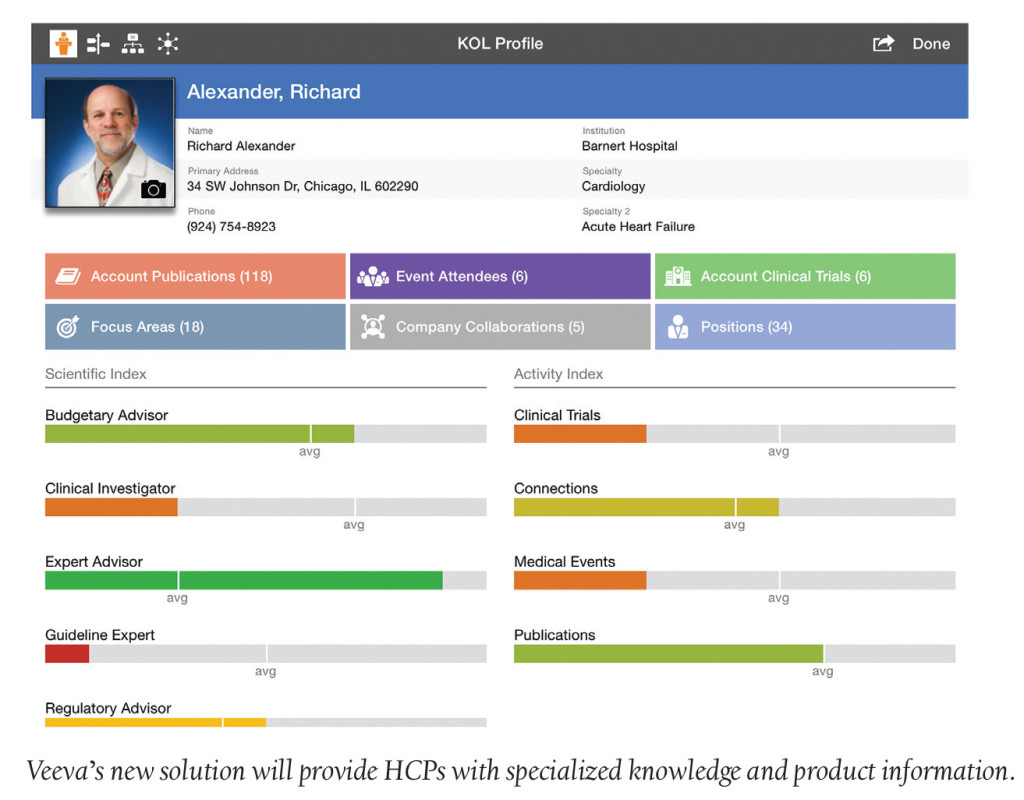 Veeva Systems unveiled Veeva Medical CRM, a cloud-based solution that enables key opinion leader (KOL) visibility and improved engagement. Armed with the right information aligned to interests and product lifecycle needs, field medical teams can drive informed scientific interactions and build deeper, more trusted stakeholder relationships.
Veeva Systems unveiled Veeva Medical CRM, a cloud-based solution that enables key opinion leader (KOL) visibility and improved engagement. Armed with the right information aligned to interests and product lifecycle needs, field medical teams can drive informed scientific interactions and build deeper, more trusted stakeholder relationships.
This is good news as the number of medical science liaisons (MSLs) is expected to grow globally by 20% during the next two years, according to a 2015 study by MSL Insights. Largely driven by the rise of complex drug products, this growth reflects the increasing need by HCPs for more specialized knowledge and product information. Hindered by dozens of systems, however, MSLs struggle to quickly and accurately identify, profile and engage KOLs. Veeva’s solution is designed to enable better stakeholder planning and engagement.
Discoveries/Innovations: Neon Green Band-Aids Could Keep Patients Healthy
Scientists have developed a gel substance to be inserted into a band-aid that will make it turn neon green if a bacterial infection sets into a wound. Infections are common when healing from even minor injuries and could become dangerous quickly. This new band-aid, developed by a team at the University of Bath led by biophysical chemistry professor Toby Jenkins will signal a patient about an infection. Nipping a bacterial infection in the bud is the key to preventing illness. Jenkins is particularly interested in using the technology for child burn victims. Children with burns are often over treated with antibiotics since infections can be severe. However, this often leads to bacterial infections that may also be dangerous. This technology could help medical professionals find a balance to keep children healthy.
FDA Update
Approvals
Adzenys XR-ODT (Amphetamine Extended-Release Orally Disintegrating Tablet) received approval for the treatment of ADHD in patients six years of age and older. Manufactured by Neos Therapeutics, this medication is novel in that it is the first of its kind and is taken once daily.
Merck received approval for Zepatier (elbasvir and grazoprevir), a new and highly anticipated hep C treatment designed for the treatment of adults. Specifically targeting HCV GT4 infections, the medication is taken once daily to treat chronic infection in patients with end stage renal failure disease who are on hemodialysis.
Onzetra Xsail (sumatriptan nasal powder) manufactured by Avanir Pharmaceuticals recently received FDA approval as the first breath-powered intranasal delivery system for the acute treatment of migraines in adults. A fast-acting powder, the drug delivery system treats migraines with or without migraine aura.
The FDA also approved the drug-device combination ZEMBRACE SymTouch from Dr. Reddy’s Laboratories for the treatment of acute migraine episodes, with or without aura, in adults who are inadequately managed with existing treatment regimens.
Eisai received approval for Halaven (eribulin mesylate) a type of chemotheraphy for the treatment of a specific type of soft tissue sarcoma, liposarcoma, for patients who have received prior chemotherapy containing an anthracycline drug.
Orphan Drug Designation
Immunocore was granted Orphan Drug Designation for IMCgp100, a treatment for uveal melanoma. Uveal melanoma occurs when cancer cells form in the eyes. Currently, no effective treatments are available for this form of cancer.





