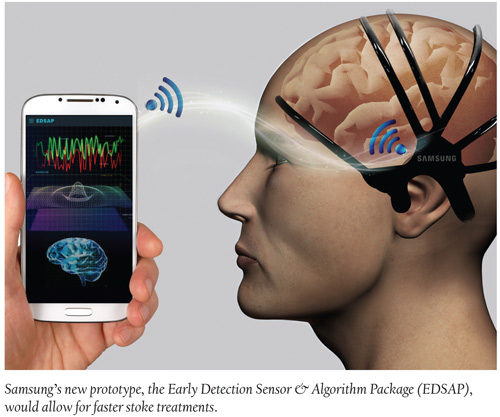
TeleMed Texts: New Prototype Detects Stroke Likelihood Within a Minute
Fifteen million people across the world suffer from stroke each year with roughly 66% of these strokes resulting in death or permanent physical disabilities, according to the World Health Organization. A new prototype solution from Samsung, however, the Early Detection Sensor & Algorithm Package (EDSAP), can, according to the company, detect a stroke quickly allowing for faster treatment. EDSAP, an easy-to-wear device, wirelessly transmits brainwave data to a mobile app and can detect—within 60 seconds—the likelihood of a stroke using sensors and an algorithm. And if EDSAP is worn for longer durations it could also provide additional information related to neurological health, such as stress, anxiety and sleep patterns. The device is able to analyze brainwaves much faster than the standard hospital brainwave monitors—which can take about 15 minutes—and scan brainwaves in more comprehensive detail thanks to the highly conductive rubber-like material used on the sensors. The team behind the project also plans to assess other applications for this technology, such as with electrocardiograms.
Discoveries/Innovations: Breakthrough in Orthodontic Treatment
The first and only noninvasive FDA-cleared medical device of its kind, AcceleDent, is said to speed up orthodontic treatment by as much as 50%. According to manufacturer, OrthoAccel Technologies, Inc., the new hands-free device is groundbreaking in that, unlike traditional orthodontic techniques, its pulsatile force accelerates bone modeling and remodeling in the craniofacial region. Based on significant results among patients, the company states that some of the nation’s leading orthodontists claim AcceleDent is the only fast, safe and gentle way to accelerate orthodontic treatment.
“More patients are asking their orthodontists about this accelerated treatment and orthodontists are eager to offer AcceleDent due to positive patient results,” said OrthoAccel’s President and CEO, Mike Lowe, in a statement. Ultimately, he adds, the company’s goal is to improve patient journeys while creating healthy, beautiful smiles. The device is now available in 2,000 orthodontic locations across the U.S.
Doctor Docs: Walgreens Makes Virtual Doctor Visits Possible
Walgreens recently expanded its telehealth services to offer virtual doctor visits on its mobile app and provide 24/7 access to U.S. board-certified doctors through MDLive, the nation’s leading provider of telehealth services. Typically, a 10- to 15-minute MDLive virtual visit costs $49. According to the Walgreens, the mobile app is not meant to replace yearly visits to a primary doctor, but is meant for non-emergency health conditions including upper respiratory tract infections, earaches, sore throats and rashes. After a virtual visit, physicians can ePrescribe medication.
The virtual service is currently available in iOS and Android for users in California and Michigan. Through the app, Walgreens and MDLive are placing more responsibility for healthcare into their customer’s hands.
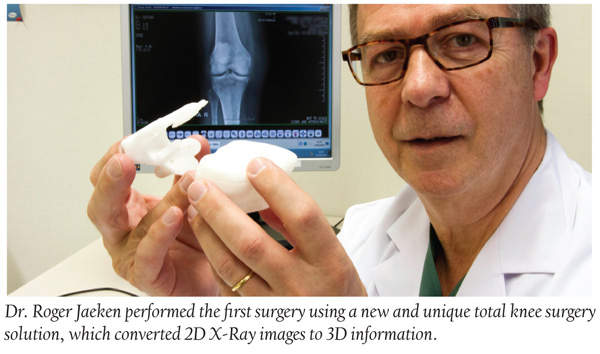
Med Device: Total Knee Surgery Solution Eliminates CTs and MRIs
Orthopedic surgeon, Dr. Roger Jaeken of the AZ Heilige Familie (Holy Family General Hospital) in Reet, Belgium, performed the first total knee surgery using Materialise’s X-ray knee guide solution—the next generation of total knee surgical planning and guiding tools. The new solution allows surgeons to preplan knee replacement. The X-ray device is unique, according to the company, because it does not require CT or MRI scans of the patient’s knee. Instead it converts 2D X-ray images to 3D information and solutions.
“The ability to work from X-ray images will make the surgical preplanning process even more efficient,” according to Dr. Jaeken. Patients, he adds, will not only no longer need to undergo time-consuming CT or MRI scans, but more patients will also become eligible for preoperative planning.
Therapeutic Talk: New MRI Technique Improves Prostate Cancer Detection
In 2014, prostate cancer was the leading cause of newly diagnosed cancers in men, according to the American Cancer Society (ACS), and the second leading cause of cancer death in men. This year, the ACS estimates another 220,800 new prostate cancer diagnoses and 27,540 deaths from the disease in the U.S.
However, in the January 6, 2015 issue of journal Prostate Cancer and Prostatic Diseases, a team of scientists and physicians from the University of California, San Diego School of Medicine and University of California, Los Angeles described a new MRI technique called restriction spectrum imaging-MRI or RSI-MRI, which produces measurably better results in detecting prostate cancer tumors than the current standard, contrast-enhanced MRI. While the current detection/diagnosis standard involves injecting patients with a contrast agent to highlight blood flow, the new RSI-MRI focuses on water diffusion within tumor cells and corrects for magnetic field distortions.
According to David S. Karow, MD, PhD, assistant professor of radiology at UC San Diego and the study’s author, the technique will allow doctors to more precisely and effectively determine treatment.
FDA Update
New Deputy Commissioner Named For Medical Products and Tobacco
Duke University cardiologist Robert Califf will become the Deputy Commissioner for Medical Products and Tobacco at the end of February. In his new role, Califf will serve as the executive leader of the Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health and the Center for Tobacco Products.
Recent Approvals
The FDA approved Teva’s generic version of AstraZeneca’s blockbuster Nexium (esomeprazole) for the treatment of gastroesophageal reflux disease in adults and children age one and older. Teva’s new generic version of Nexium is the first approved in the U.S. EnteroMedic’s VBLOC vagal blocking therapy recently received approval as an obesity treatment that works by targeting the nerve pathway between the brain and the stomach. Intact Medical Corporation received expanded marketing clearance for its Intact technology, which allows surgeons to remove intact breast lesions up to 30mm in diameter in less than 20 seconds.
Priority Review
Janssen Research & Development was granted Priority Review of an atypical antipsychotic (paliperidone palmitate) to treat schizophrenia in adults. The treatment is designed as the first and only long-acting atypical antipsychotic with a dosing schedule of just four times a year.
Fast Track Designation
Celator Pharmaceuticals, Inc. received a fast track designation for CPX-351, a treatment for secondary acute myeloid leukemia in elderly patients. The Fast Track designated product could be eligible for priority review if supported by clinical data at the time of NDA submission.