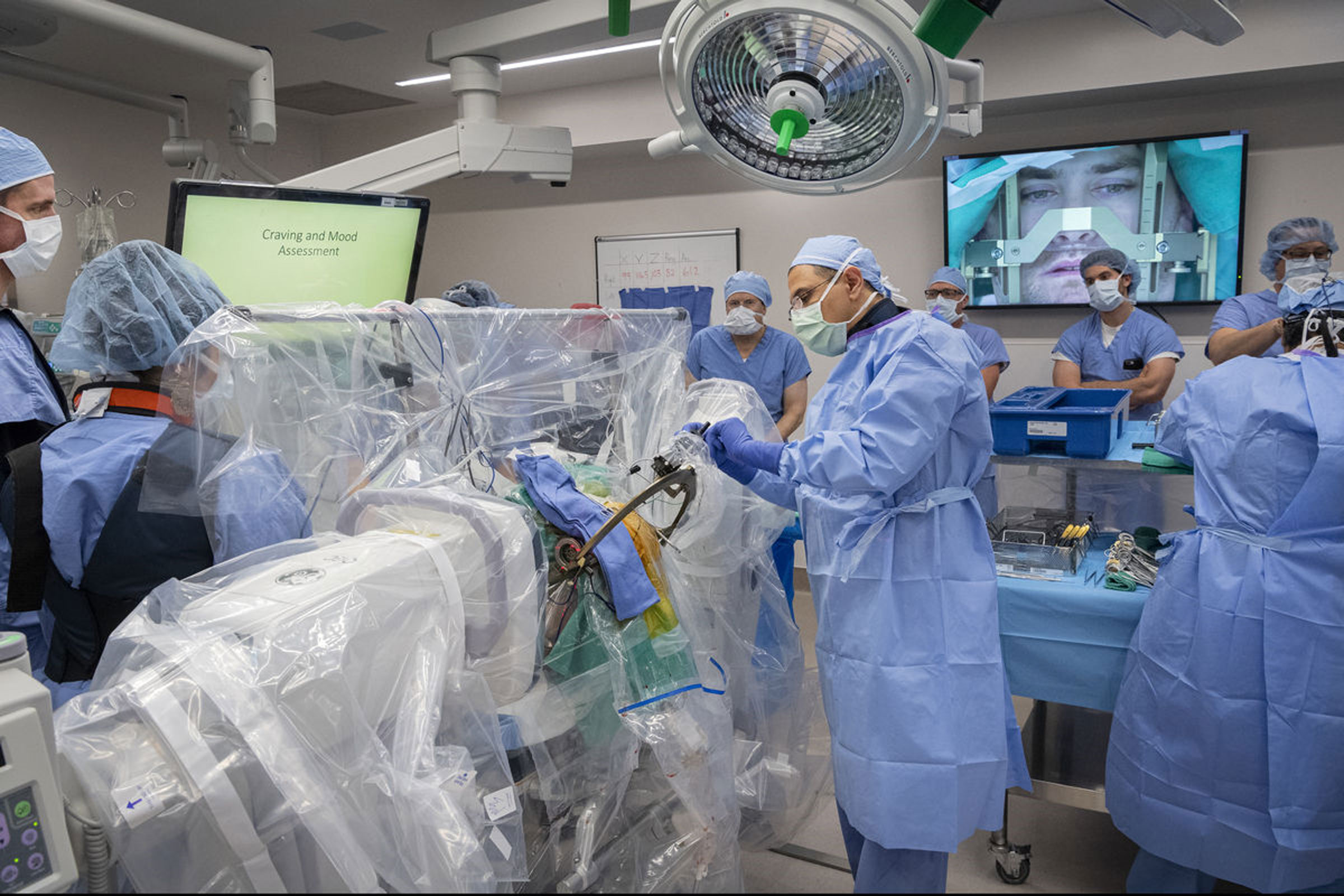
Therapeutic Talk: Brain Stimulation Could Be the Answer to Opioid Addiction
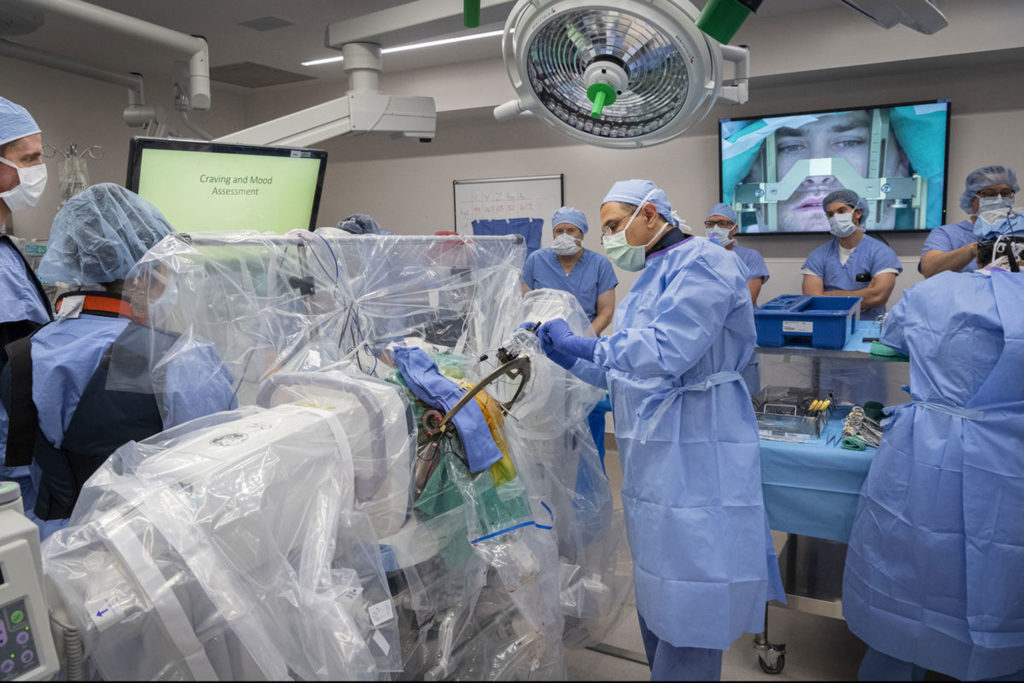
For the first time in the U.S., a clinical trial is underway to test the effectiveness of deep brain stimulation (DBS) on patients suffering from treatment-resistant opioid use disorder. The West Virginia University Rockefeller Neuroscience Institute and WVU Medicine team successfully implanted a Medtronic DBS device in the addiction and reward center of the brain of the first of four participants. This requires a surgical procedure to implant tiny electrodes into specific brain areas to regulate the structures involved in addiction and behavioral self-control. All of the participants have failed to respond to care from WVU Medicine’s comprehensive inpatient, residential, and outpatient treatment programs that include medication, as well as psychological and social recovery efforts.
“Despite our best efforts using current, evidence-based treatment modalities, a large number of patients simply don’t respond. Some of these patients remain at very high risk for ongoing catastrophic health problems and even death. DBS could prove to be a valuable tool in our fight to keep people alive and well,” James Berry, DO, interim chair of the WVU Department of Behavioral Medicine and Psychiatry and Director of Addiction Services at RNI, stated.
The National Institute on Drug Abuse is funding the clinical trial in West Virginia, where drug overdose deaths involving opioids is the highest in the U.S. The study not only aims to help participants overcome opioid addiction, but will allow researchers to gather data on the addiction centers of the brain and develop new technologies in the future.
Trend Setting: Janssen Makes Clinical Trials Digital
Janssen has launched a first-of-its-kind clinical trial that is completely virtual. Using wearables and virtual contact with participants, the CHIEF-HF is the first-ever completely decentralized, mobile, indication-seeking clinical study. The trial’s aim is to more quickly and efficiently gather and analyze real-world data, fast-tracking the study and results for assessing the effectiveness of Invokana (canagliflozin) in adults with heart failure, with or without type 2 diabetes. CHIEF-HF (Canagliflozin: Impact on Health Status, Quality of Life, and Functional Status in Heart Failure), will examine the use of the drug compared to placebo in regards to quality of life.
“Traditional clinical trials are undeniably essential in medical research but are often long and costly. Through the CHIEF-HF study, we are exploring how we can harness technology that consumers already have at their fingertips, including smartphones and wearable devices, to change this paradigm,” stated Paul Stoffels, MD, Vice Chairman of the Executive Committee and Chief Scientific Officer at Johnson & Johnson. “Through this virtual trial approach, we hope to make clinical studies more inclusive, faster, and more cost-effective so that we can deliver innovative solutions to the people who need them.”
Investigators on CHIEF-HF will collect participant-reported outcomes through app-based clinical questionnaires and physical activity data as logged by a smartphone app and a wearable activity device that records daily step count and stairs climbed.
Patient Pages: Are Patients Engaging with Digital Health Records?
Efforts to adopt electronic health records in U.S. hospitals may not be as useful as we once thought. A study finds that only one in 10 patients with access to EHRs actually engaged with the digital documents once discharged. The study team from Oregon Health and Science University-Portland State University School of Public Health found that 95% of hospitals give their patients electronic access, but only 10% of discharged individuals take advantage of it. The study included 2,410 hospitals nationwide between 2014 and 2016.
“This suggests that there is significant room for improvement in engaging patients with their information electronically,” the study’s lead author, Sunny Lin, writes in Health Affairs.
“Overall, our findings suggest that policy efforts have failed to engage a large proportion of patients in the electronic use of their data or to bridge the ‘digital divide’ that accompanies healthcare disparities.”
Starting in 2011, the Centers for Medicare and Medicaid Services (CMS) launched a national program with financial incentives for hospitals to adopt electronic medical records in an effort to speed the adoption and use of health information technology. As part of this program, in 2014 hospitals were required to provide patients with access to digital records. The study team found that low income hospitals provide patients with this service less often. Future studies might ask how hospital characteristics or behaviors can impact patient engagement with their digital records as well as how access to digital records directly affect patient outcomes.
Doctor Docs: AMA Takes on Racial and LGBTQ Issues

At this year’s Interim Meeting, the American Medical Association (AMA) approved policies that will affect the LGBTQ and racial minority communities. First, the AMA will develop model state legislation and advocate for a federal ban on so-called reparative or conversion therapy for sexual orientation or gender identity. The AMA has long denounced the practice as unscientific and continues to cite depression, post-traumatic stress disorder, and suicidal thoughts and attempts as the only potential outcomes to conversion therapy. While the AMA takes a clear stance on ending this practice in the U.S., currently no states have banned conversion therapy for adults.
Second, the AMA is strengthening its existing policy to promote inclusive gender, sex, and sexual orientation options in medical documentation. A lack of fully inclusive EHR documentation is a barrier to providing quality care to the transgender community. The AMA advocates for the voluntary inclusion of a transgender patient’s preferred name and clinically relevant sex-specific anatomy in medical documentation. “Without this information, transgender patients and their specific healthcare needs cannot be identified or documented and the health disparities they experience cannot be addressed—and the provision of important healthcare services may not be delivered,” states AMA board member William Kobler, MD. The organization also encourages medical schools to continue to periodically reassess education on health issues related to sexual orientation and gender identity.
Finally, the organization adopted a policy supporting measures to eliminate racial pay disparity in medicine. This includes working with stakeholders to study effective ways to increase the transparency and accountability of physician earnings. The AMA supports physician access to information including but not limited to the salaries, race, and ethnicity of physicians.
Med Device: New Contacts Correct Child Nearsightedness

CooperVision developed and received FDA approval for the first-ever contact lenses to slow the progression of nearsightedness in adolescents 8 to 12 years old. The MiSight lens is a soft, single-use contact, designed to be discarded at the end of each day. Nearsightedness, or myopia, is caused by a gradual elongation of the eyeball from front to back. These contacts are proven to reduce elongation by 52% over three years.
“We can’t overstate the importance and potential impact of this landmark decision on children’s vision, especially considering the rise in myopia’s severity and prevalence in the U.S. and worldwide,” CooperVision President Daniel McBride said in a statement. “Eye care professionals who embrace this breakthrough approach will improve the quality of life and eye health for so many children.”
The FDA also claims that the MiSight lenses could help prevent other eye-related issues that are common for children with myopia. Children who develop severe myopia are more likely to be susceptible to cataracts or a detached retina during adulthood.
FDA Update
Drug Approvals
The FDA approved SK Life Science’s Xcorpi (cenobamate tablets) to treat partial-onset seizures in adults. The difficult-to-control condition occurs during episodes of abnormal electrical activity in the brain. Partial-onset seizures occur in a limited portion of the brain.
Under the initiative Project Orbis, the FDA Oncology Center of Excellence has approved Calquence for adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). AstraZeneca’s drug was approved via a joint decision between the FDA, the Australian Therapeutic Goods Administration, and Health Canada. Project Orbis provides a framework for concurrent submission and review of oncology drugs among international partners.
Pfizer’s Abrilada has been approved as the fifth biosimilar to Humira. This is also the 25th biosimilar to be approved overall by the FDA. The drug is approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The FDA approved Novartis’ Adakveo (crizanlizumab-tmca), the first targeted therapy for a painful complication stemming from sickle cell anemia known as vaso-occlusive crisis. This common and painful episode occurs when blood circulation is obstructed by sickled red blood cells. Adakveo can helps to reduce the frequency of vaso-occlusive crisis.
Med Device Approvals
Myriad Genetic Laboratories received approval for myChoice CDx laboratory kit for detecting homologous recombination deficiency (HRD) status. For patients with ovarian cancer, a positive HRD status means there are mutations in BRAC1 and BRCA2 genes or a high Genomic Instability Score (GIS) which indicates the DNA cannot be repaired. A physician takes a sample of the patient’s tumor and requests testing with the myChoice lab kit to recommend ovarian cancer treatment.