Doctor Docs: BD’s New Platform Spots Opioid Diversion
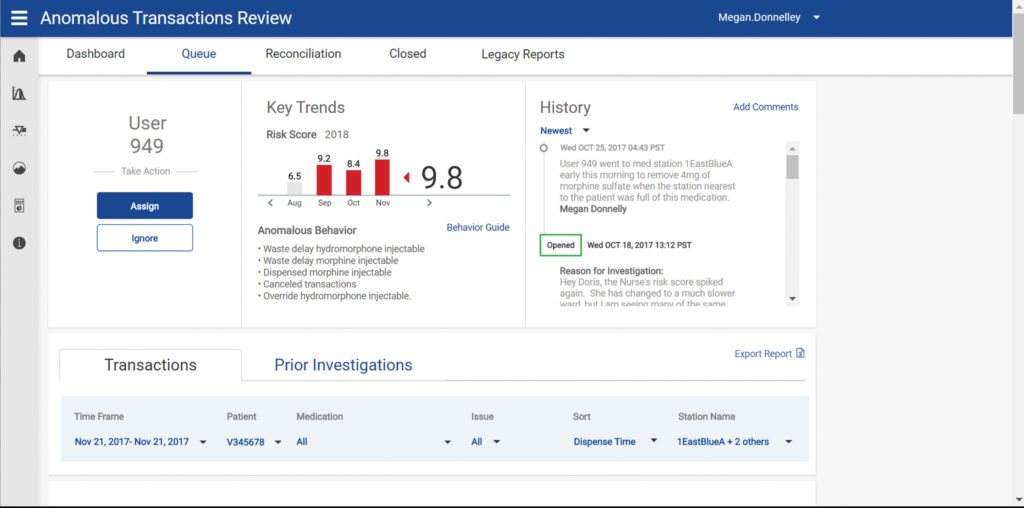
The national opioid crisis has brought awareness to the role and impact prescription drugs have in drug misuse and abuse. This phenomenon largely originates and is fueled by drug diversion within healthcare settings. Diversion of drugs, for personal use or illegal distribution, can cause significant financial loss and potentially impact care to patients and staff safety. To combat this crisis, BD launched a new software application called the BD HealthSight platform, which is designed to support enterprise-wide medication management, helping hospitals and health systems identify drug diversion.
Medication management is incredibly complex. There are many points at which diversion may occur and many methods of diversion. An enterprise-wide approach is therefore critical to meeting these challenges and includes an interoperable combination of connected technologies and robust analytics. To accomplish this, BD HealthSight diversion management was built as a hosted, cloud-based application that assists with drug diversion investigations by creating an investigation workflow to monitor, triage, and assign potential diversion cases to specific investigators.
The Challenge for Health Systems
“Medication diversion is a growing and complex challenge for hospitals and health systems. We believe that to best address this challenge, a holistic approach to medication management that includes a combination of connected technologies and robust analytics is required,” said Ranjeet Banerjee, Worldwide President of Medication Management Solutions for BD. “Specific cases of diversion can be difficult to detect, and the impact can be devastating from a patient and healthcare worker safety standpoint. The new BD HealthSight diversion management application is designed to address the unique challenges associated with diversion by tracking patterns and risky behavior to help identify diverters as early as possible.”
Compared to traditional, statistically based analytical tools that only look at amounts dispensed to identify potential diversion, BD uses machine learning algorithms and multiple dispensing behaviors—such as overrides, canceled transactions, delays in dispensing, administering, and wasting medications—to unveil clinicians whose behavior indicates higher risk for diversion.
TeleMed Text: CSF joins Backpack Health to Offer Health Management Tools
The Children’s Scoliosis Foundation (CSF) announced a partnership with Backpack Health that will provide families of children with a scoliosis diagnosis with the health information technology company’s data management application, providing patients and their caregivers with one centralized platform to store, organize, track, and share health information. The cloud-based mobile app offers pediatric scoliosis patients and their caregivers a place to manage health data and connect with the global scoliosis community.
Scoliosis, a sideways curvature of the spine that is most common in late childhood and early adolescence, affects 2% to 3% of the population, and the cause is currently unknown. Severe scoliosis can reduce the amount of space in the chest, making it harder for the heart and lungs to function properly.
Managing Medical Records
“Families will travel all over the world to find the right treatment for their child’s scoliosis. They have the added burden of carrying stacks of medical records with them each time they travel, which can be even more overwhelming when caring for more than one child with scoliosis,” says Anne Knox, MS, CEO of The Children’s Scoliosis Foundation in a statement. “Backpack Health’s app helps families easily manage all medical information and provides the ability to share information securely with multiple providers. Additionally, Backpack Health’s patient empowerment platform allows us to manage deployment to scoliosis clinics around the world in one solution.” Backpack Health also offers patients the ability to participate in the collection of de-identified data that may serve to support researchers.
“CSF serves as an important resource for families of children with scoliosis. We are excited to partner with CSF and offer the scoliosis community further support via our digital tools,” says Jim Cavan, CEO and Founder of Backpack Health, in a statement. “Together Backpack Health and CSF will offer patients full control of their own health journeys.”
DC Dispatch: FDA Med Device System Overhaul to Begin

For decades, the FDA’s process for reviewing and approving medical devices has not changed, much to the chagrin of doctors and patients alike. After years of criticism from experts who say systems fail to catch problems with risky implants and related products, the FDA promises to make sure new medical devices reflect up-to-date safety and effectiveness features, rather than allow manufacturers to launch new products based on similarities to existing products. Unlike new pharmaceuticals, which are tested in patient studies, the increasingly complex medical devices often barely resemble the decades-old “predicates” they claim to reference but are still able to get speedy approval.
The declaration comes after an investigation, led by the International Consortium of Investigative Journalists, found that more than 1.7 million injuries and nearly 83,000 deaths suspected to be linked to medical devices had been reported to the FDA over a 10-year period. “We believe that it’s time to fundamentally modernize an approach first adopted in 1976,” FDA Commissioner Scott Gottlieb, MD said in a statement. This modernization, which could include anything from new guidelines for manufacturers to action taken by Congress, will likely take years to implement. While this overhaul could lead to safer devices, it may also be faced with serious counter lobbying efforts from the device industry, slowing progress and preventing true reform.
Discoveries/Innovations: Revolutionary Step in Cartilage Health
Until now, osteoporosis medications have all failed to make a real difference to those suffering with the illness because they disappear from the affected joint before they can even penetrate the damaged tissue. Researchers at MIT have found a way to bypass this tissue barrier with a round-shaped “nanocarrier.” The nanocarrier is a molecule that contains tentacle-like structures positively charged at their tips, allowing them to bind to the negatively charged cartilage for diffusion. “We found an optimal charge range so that the material can both bind the tissue and unbind for further diffusion, and not be so strong that it just gets stuck at the surface,” Brett Geiger, the lead investigator, said in a statement.
The nanocarrier is merely a vehicle intended to bring a medication deeper into the tissue so it has time to affect the difficult-to-heal cartilage. The MIT scientists used an experimental drug called insulin-like growth factor 1 (IGF-1), which is a protein that stimulates the growth of many tissues, including cartilage. In rats with osteoarthritis, they found that the nanocarrier-attached IGF-1 had a half-life of about four days, which is 10 times longer than IGF-1 alone. The team wrote that this translated into better cartilage restoration, and reductions in joint inflammation and bone spurs, as compared to IGF-1 alone.
With so many recent arthritis therapy failures, the MIT researchers hope their technology could one day be used to improve arthritis drugs that treat the underlying cartilage problem.
Brand Beat: Pharma Marketers Battle Amazon
 While Amazon is the third largest online platform for digital ads, pharmaceutical advertisements are nowhere to be seen. Retailers dominate the marketspace since only OTC medications can be sold online, whereas prescription advertising would be futile. However, OTC medications do make up a large portion of many drug manufacturers’ consumer divisions. Without advertising on Amazon, drug makers are missing out on the 50% market share of the retail giant’s U.S. sales. Amazon has already launched a Basic OTC care line as it primes itself to enter the pharmaceutical market at a still unknown angle. At this point, pharma marketers with OTC brands should be figuring out their own strategies for Amazon, according to Wes MacLaggan, Senior Vice President of Marketing at ad management platform Marin Software, which works with OTC drugmakers including Pfizer and GlaxoSmithKline.
While Amazon is the third largest online platform for digital ads, pharmaceutical advertisements are nowhere to be seen. Retailers dominate the marketspace since only OTC medications can be sold online, whereas prescription advertising would be futile. However, OTC medications do make up a large portion of many drug manufacturers’ consumer divisions. Without advertising on Amazon, drug makers are missing out on the 50% market share of the retail giant’s U.S. sales. Amazon has already launched a Basic OTC care line as it primes itself to enter the pharmaceutical market at a still unknown angle. At this point, pharma marketers with OTC brands should be figuring out their own strategies for Amazon, according to Wes MacLaggan, Senior Vice President of Marketing at ad management platform Marin Software, which works with OTC drugmakers including Pfizer and GlaxoSmithKline.
Much like the way Google works, pharma marketers can purchase brand banners and sponsored product listings, both of which appear above organic search results. With a majority of Americans turning to Amazon first for practically any type of purchase, drug makers can’t ignore even these small opportunities. “Rather than trying to avoid it entirely, it’s advisable to figure out how to cooperate and work within the system as best as possible because customers are very loyal to Amazon. If you’re not on the channel, people will find other products to use in your category,” MacLaggan said in a statement. He advises marketers to create robust and differentiated product information pages, creating a positive buying experience so the product reviews many potential consumers rely on are as influential as possible.
FDA Update
Drug Approvals
Pfizer receives FDA approval for Daurismo, a new treatment for newly-diagnosed acute myeloid leukemia (AML), a rapidly progressing cancer that forms in the bone marrow and increases the number of abnormal white blood cells in the bloodstream and bone marrow. The tablets are used in combination with low-dose cytarabine (LDAC), a type of chemotherapy, in adults who are 75 years of age or older, or who have comorbidities that may make intensive chemotherapy impossible.
The FDA approves the first treatment specifically for fighting the rare and fatal immune disease, primary hemophagocytic lymphohistiocytosis (HLH). Novimmune’s Gamifant fights refractory, recurrent, or progressive HLH, which causes the body’s immune cells to become overactive, damaging the body’s own organs, including the liver, brain, and bone marrow.
Celltrion’s and Teva’s Truxima is the first biosimilar to Rituxan approved for the treatment of adults with non-Hodgkin’s lymphoma. Truxima meets standards that require it to have no clinically meaningful differences in terms of safety, purity, and potency to Rituxan.
Medical Devices
Ortho Clinical Diagnostics’ VITROS XT 7600 Integrated System receives FDA clearance, introducing DIGITAL CHEMISTRY technology to clinical lab management by combining Ortho’s MicroSlide technology with digital imaging capabilities, offering the potential to perform two separate lab tests simultaneously. The system provides operational improvements and heightened quality to help clinical laboratories keep pace with an evolving global healthcare environment, without requiring additional lab space.
The GORE Carotid Stent, manufactured by W. L. Gore & Associates, Inc., is used to re-open narrowed regions of the carotid arteries in the neck that supply blood to the brain. The FDA-approved implant is made of nickel-titanium alloy (nitinol) tubing, laser-cut into a mesh shape, used with a protection device (GORE Embolic Filter) that filters blood flow at the treatment site to capture and remove particles that may come loose during the procedure.







