Discoveries/Innovations: T-cells Discovery Can Help Pick Best Cancer Treatments
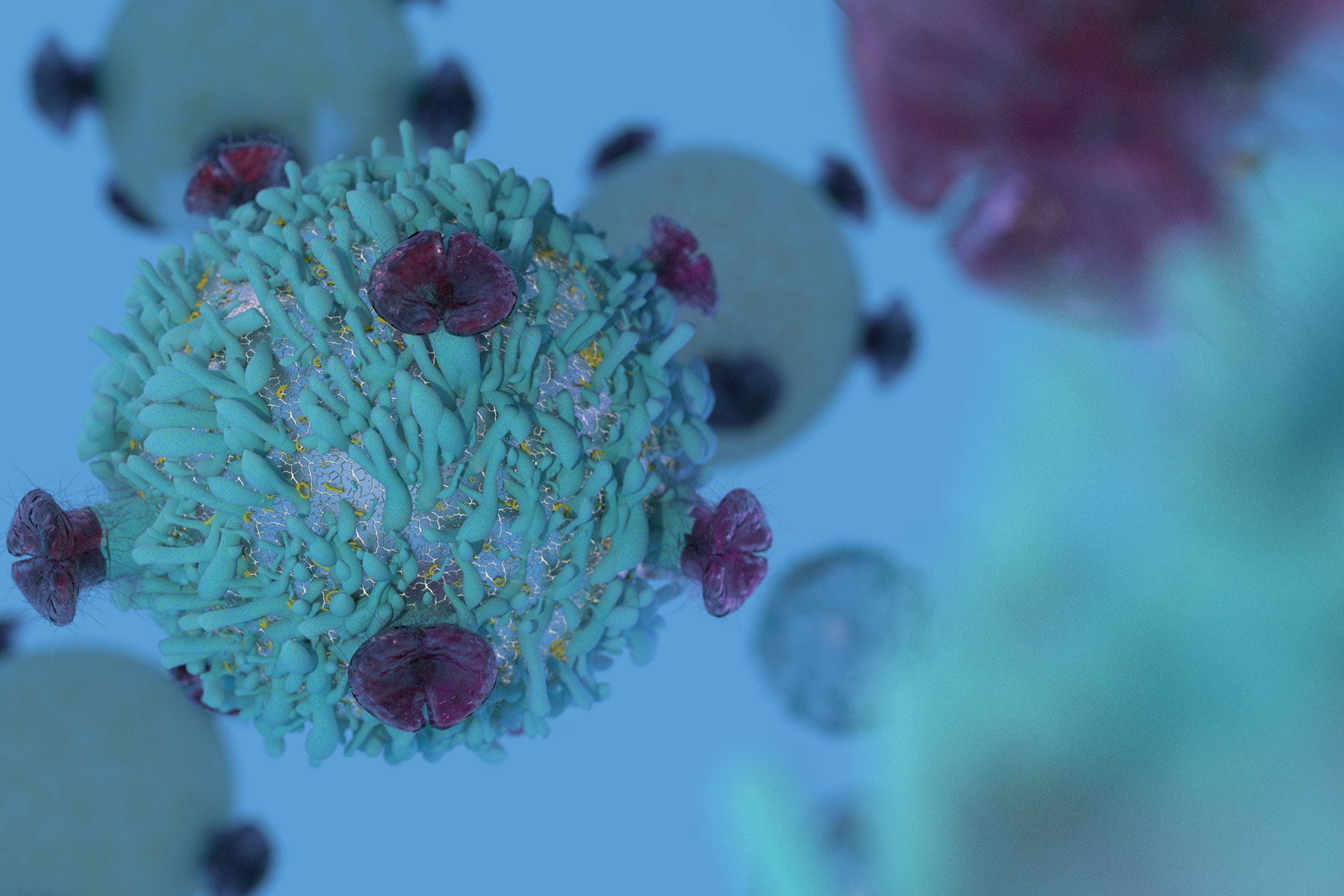
UK researchers have found that lung cancer patients with large amounts of specific T-cells are 34% more likely to survive. These T-cells cluster together in tissues not only to act as protectors, but also to produce molecules that attack tumors. Testing for levels of these cells could help doctors identify which patients will benefit most from immunotherapies that help to ramp up the body’s attack on the cancer.
“These are hugely exciting results,” Professor Christian Ottensmeier, Cancer Research UK scientist at the University of Southampton, said in a statement. “For the first time, we have a real indication of who might benefit from a particular drug before we make treatment decisions. So far when we use immunotherapy we do not know if a patient will benefit. The new findings are a big step towards making this exciting treatment much more predictable. The research explains why some patients might be more receptive to treatments and help personalize therapies in the future.”
TeleMed Text: App to Battle Insufficiencies in Mental Healthcare
There are more that 50 million Americans suffering from mental illness—and yet it is still a stigmatized disease that is not given proper attention in the healthcare industry. Thirty million of diagnosed patients do not have medical coverage for mental illness at all. LARKR, a revolutionary healthcare platform, has just launched a smartphone app that allows video conference between patient and provider. The convenient app decreases the hurdles of price, accessibility, and stigma for mental illness patients, especially those not insured due to the gap in healthcare.
Low cost, 50-minute video sessions can be accessed nationwide and this is the first app of its kind to work with patients under the age of 18—the U.S.’s most at-risk demographic. It also allows for multi-user, multi-location sessions for group and couple therapy and focuses on impactful talk therapy over a video conference, rather than impersonal phone calls or prescribing medication. LARKR was co-founded by Shawn Kernes, a former executive at StubHub and eBay, and offers only U.S. licensed therapists.
Medical Devices: Game Changing Saliva Test for Oral Cancer
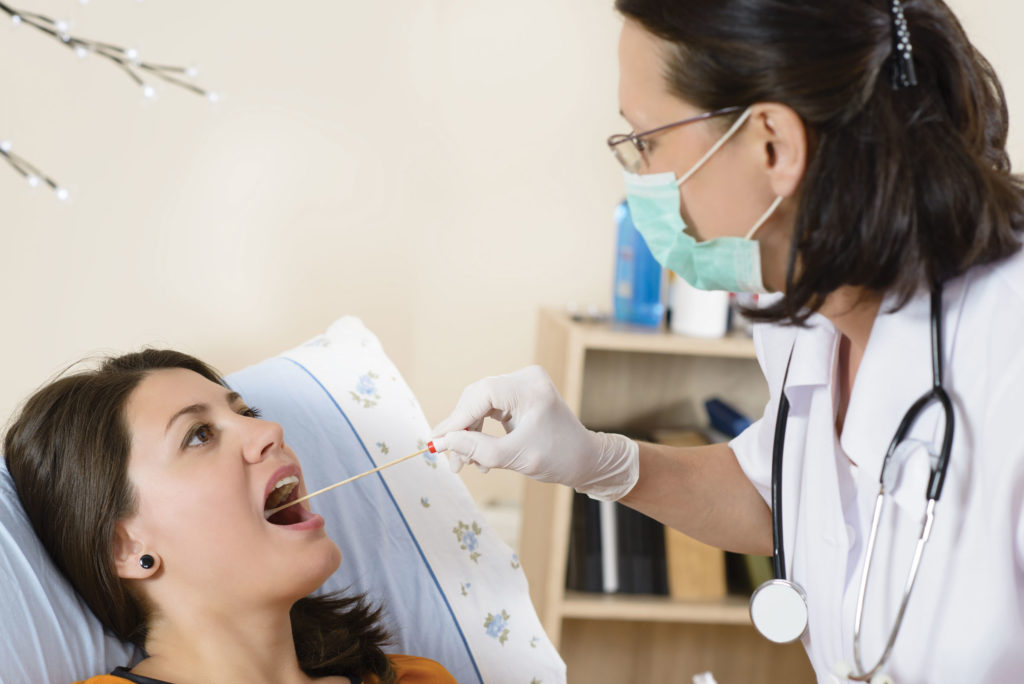
MDxHealth will collaborate with Queensland University of Technology (QUT) to develop a liquid biopsy for early detection of oral cancers. The importance of the project lies in the fact that catching oral cancer in its early stages is crucial for survival and no U.S. screening guidelines or blood/saliva-based diagnostic tests exist for head and neck cancers. There are 20,000 global incidences of oral cancer annually, which often turn fatal since symptoms become noticeable in advanced stages.
“A simple and fast point of care test could help dentists to rule-out oral cancer while you have your dental checkup,” said Jan Groen, PhD, CEO of MDxHealth. “This scientific collaboration is an opportunity for both MDxHealth and QUT to leverage existing know-how to develop a ground-breaking oral cancer test that will improve overall survival for thousands of patients.”
Under the terms of the agreement, MDxHealth and QUT bluebox will both evaluate and develop the test and MDxHealth will have the first option to license the commercial diagnostics rights.
Therapeutic Talk: Another Step Towards Treating Hepatitis B
Researchers at the Universities of York and Leeds have discovered that the genetic code in Hepatitis B produces a protective casing in which new infectious virus particles are formed. This system is necessary to properly assemble new genetic material and therefore, spread. While there is a vaccine for the disease, no cure exists for the two million already infected globally who often die from resulting liver cancer.
“We often compare the disease to HIV due to the way in which the virus is passed from person-to-person, but unlike HIV there are no effective drugs to improve quality of life outcomes,” Professor Peter Stockley, a structural virologist from the University of Leeds, said in a statement. “Now that we know how the virus assembles, we can interrupt the interactions with the RNA signals—a bit like when a twig catches the sprocket on a bike, knocking the chain off.” The researchers are working with the National Institutes of Health in the USA to identify potential drug candidates that are capable of breaking the link between RNA and proteins, which should halt viral replication.
FDA Update
Drug Approvals
Genentech’s combination of rituximab and hyaluronidase human for adult patients with follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia was recently granted FDA approval. Rituxan Hycela provides patients with a subcutaneous route of rituximab administration that shortens the administration time to five to seven minutes as compared to intravenous infusion that can take several hours.
Bevyxxa, Portola’s drug for venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness, received FDA approval. The pills reduce risk for thromboembolic complications due to moderate or severe restricted mobility and other risk factors for VTE.
Melinta Therapeutics received FDA approval for their drug Baxdela, used to treat adult patients with acute bacterial skin and skin structure infections, including hospital-treated skin infections in oral and IV formulations. As immunity to existing antibiotics continues to rise in the U.S. population, this drug has become an important new weapon against MRSA and other serious pathogens.
The FDA approved Symjepi, Adamis Pharmaceuticals Corporation’s epinephrine injection for emergency treatment of allergic reactions. This provides adults with a new EpiPen alternative.
Medical Device
Blue Spark Technologies received a patent for their wearable, TempTraq. The device continuously senses, records, and sends body temperature to smartphones for up to 48 hours. TempTraq is a non-invasive solution for doctors and nurses who need a continuous, smarter way to track, log, and respond to fevers quickly.