Patient Pages: Patients Want To Share Health Data
A survey conducted by the Institute of Medicine’s (IOM) Evidence Communication Innovation Collaborative revealed that adult social media users with health conditions share their health data online if it is beneficial to physicians and other patients in improving care or advancing medical knowledge. Of the 2,125 PatientsLikeMe respondents with a medical condition:
- An overwhelming majority want to share health data if it could help others in some way: 94% will share to help doctors improve care; 94% want to help other patients like them; and 92% want to share to help researchers learn more about their disease.
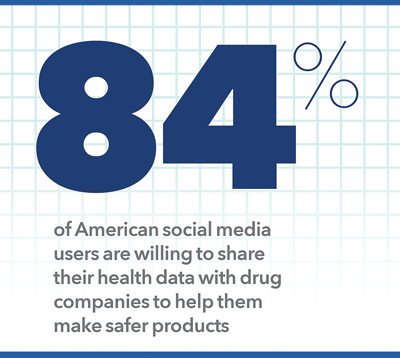
- Four out of five respondents (84%) are willing to share their health information with drug companies to help them make safer products, and 78% would do so to let drug companies learn more about their disease.
- 94% believe that their health data should be used to improve the care of future patients who may have the same or similar condition.
Discoveries/Innovations: Achilles’ Heel in Antibiotic-resistant Bacteria Found
Scientists at the University of East Anglia made a breakthrough in solving the growing antibiotic resistance of bacteria. Previously, the World Health Organization stated that antibiotic-resistance in bacteria is a global problem that makes diseases—and even common infections that have been treatable for decades—harder to treat.
According to the journal Nature, researchers investigating a particularly antibiotic-resistant class of bacteria—Gram-negative bacteria—found an impermeable lipid-based outer membrane that acts as a defensive barrier against both the human immune system and antibiotic drugs, which allows pathogenic bacteria to survive. Thus, removing the barrier makes the bacteria vulnerable to new antibiotics. The findings point to possible development of new wave of drugs that could keep bacteria from developing antibiotic resistance at all.
TeleMed Text: iPhone App Detects Melanoma
A new genetic test may help cancer patients. It’s a new app for Apple iPhone users developed by DermoScreen that is said to allow users early detection of melanoma—quickly—with an accuracy rate on par with those of dermatologists. It works by taking a phone shot of a suspicious mole or lesion, which is then processed through a software program. Within seconds, the app maker claims, you’ll know if it is likely to be cancerous. The technology does, however, require a dermatoscope attachment (a special illuminating magnifying lens) that runs about $500. Designed to provide a quick and inexpensive screening for millions of people who lack immediate access to medical specialists, the app is currently undergoing further testing at the University of Texas MD Anderson Cancer Center.
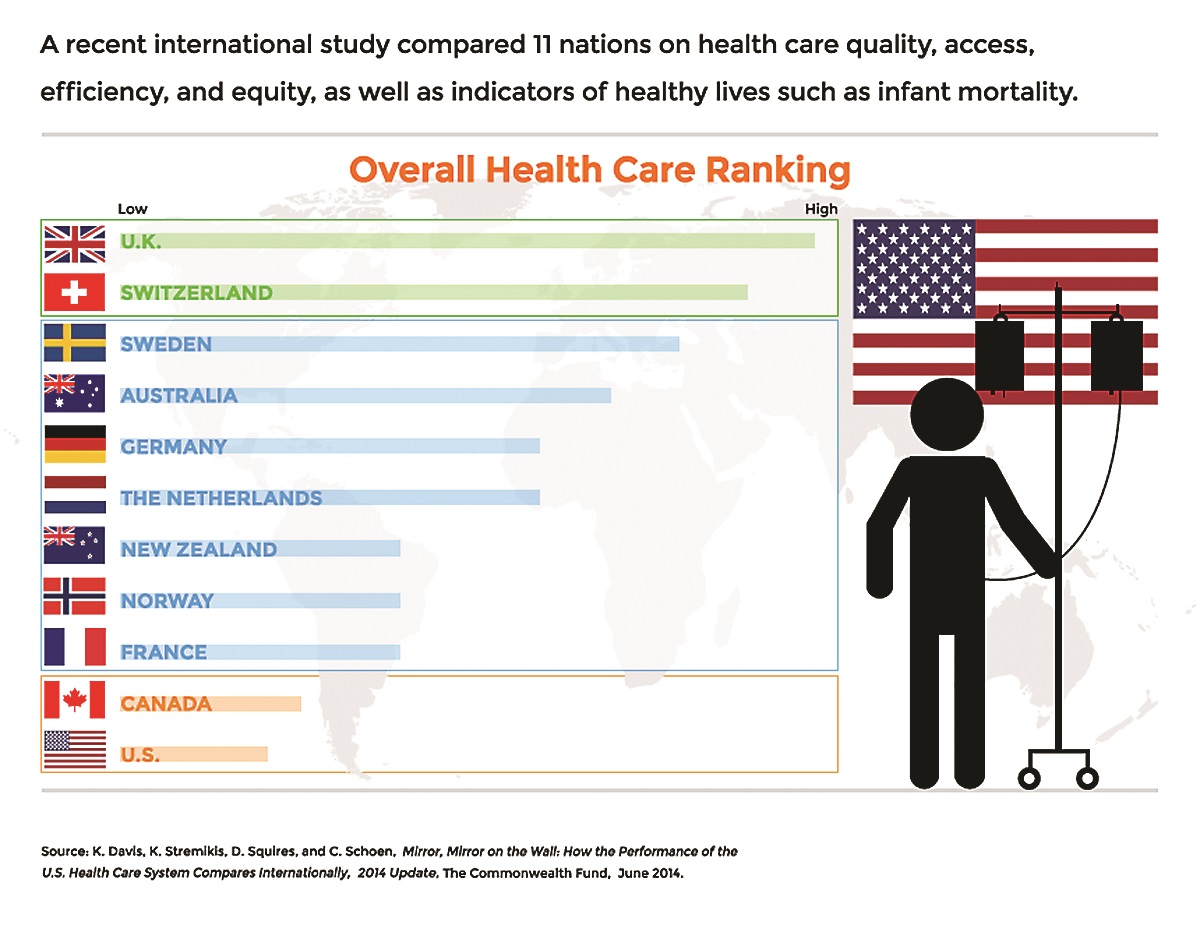
Trend Setting: U.S. Ranks Last In Healthcare—Again
The U.S. ranks last on many measures of health outcomes, quality and efficiency, says the latest Commonwealth Fund report. Among 11 nations studied (see below) the U.S ranks last and the report connects that to a lack of universal health coverage. Despite the number of newly insured patients brought in by the ACA, the U.S. underperforms on measures of access and equity between populations with above-average and below-average incomes.
Major findings of the report include:
- Quality: While improvement in recent years is reported, lower scores on safe and coordinated care pull the overall U.S. quality score down.
- Access: Americans were the most likely to report access problems related to cost.
- Efficiency: The U.S. shows poor performance on measures of national health expenditures and administrative costs as well as avoidable emergency room use and duplicative medical testing.
- Equity: One-third or more of lower-income adults in the U.S. reported they went without needed care because of costs.
- Healthy lives: The U.S. ranks last overall with poor scores on all three indicators of healthy lives—mortality amenable to medical care, infant mortality and healthy life expectancy at age 60.
FDA Update
Rare Breakthrough Designation for Biologic Agent
The first personalized cellular therapy for cancer treatment, developed by the University of Pennsylvania in collaboration with Novartis for the treatment of relapsed and refractory adult and pediatric acute lymphoblastic leukemia (ALL) uses engineered cells, known as CTL019, an investigational chimeric antigen receptor (CAR) therapy.
In early-stage trials at the Hospital of the University of Pennsylvania, 89% of ALL patients who were unresponsive to conventional therapies achieved complete remission after receiving CTL019. The Breakthrough Designation filing was submitted by the University of Pennsylvania’s Perelman School of Medicine, which has an exclusive global agreement with Novartis to research, develop and commercialize personalized CAR T cell therapies for treatment of cancers. CTL109 therapy is one of only four other biologic agents to receive this designation since the FDA created it in 2012.
FDA Approval
Novo Nordisk received FDA approval for NovoSeven RT (Coagulation Factor VIIa [Recombinant]) as the first recombinant treatment for bleeding episodes and perioperative management in patients with Glanzmann’s thrombasthenia, a rare genetic bleeding disorder with limited treatments options.
FDA Priority Review Designation
Purdue Pharma’s investigational abuse-resistant, once-daily hydrocodone bitartrate pain pill was recently granted priority review designation. The pill is designed to be more difficult to manipulate for misuse or abuse purposes.