Med Device: Neurological Disease Detected by “Mini-brains”
 Mini-brains are clumps of neurons and cells that are found in the human brain and engineered for testing drugs. These brain cells develop over the course of eight weeks and are about the size of a fly. However, the artificial brains could have a very large impact in the drug-testing field by replacing hundreds of thousands of testing animals and making drug tests more accurate.
Mini-brains are clumps of neurons and cells that are found in the human brain and engineered for testing drugs. These brain cells develop over the course of eight weeks and are about the size of a fly. However, the artificial brains could have a very large impact in the drug-testing field by replacing hundreds of thousands of testing animals and making drug tests more accurate.
The importance of this new testing method cannot be underestimated, as study leader Thomas Hartung, MD, PhD, at the Johns Hopkins Bloomberg School of Public Health, notes, “Ninety-five percent of drugs that look promising when tested in animal models fail once they are tested in humans at great expense of time and money.”
Discoveries/Innovations: Engineered Arteries for Drug Testing
A new method to make artificial blood vessels that can communicate and function normally has been developed by Duke engineers. The process is 10 times faster than the previous tissue engineering techniques and successfully replicates the inner and outer layers of natural blood vessels.
The inner layer of cells communicate with the cells of circulating blood while the outer layer is made of smooth muscle cells that regulate blood flow.
According to Nature Scientific Reports, these new blood vessels will be used to test drugs for efficacy and potential side effects as a more accurate and reliable method than using animal models.
The Tissue Chip for Drug Screening program, which is funded by the National Institutes of Health to improve drug safety and effectiveness testing, was tasked with this goal of creating 3D human tissue capable of mimicking the human body for drug trials. So far, the artificial vessels respond to anti-inflammatories and cells that regulate relaxing and constricting just as human blood vessels do. Next, the Duke researches will test how diseases affect the new blood vessels, readying them to help make strides in the entire medical research community—including the possibility of creating artificial arteries for the human body.
Doctor Docs: Single Blood Test Can Detect Heart Disease
 Doctors can now reliably test for all the inherited heart conditions through a single blood test. Professor Stuart Cook of Imperial College of London and his team have determined that studying a different set of genes makes for a quicker and much more efficient heart disease test.
Doctors can now reliably test for all the inherited heart conditions through a single blood test. Professor Stuart Cook of Imperial College of London and his team have determined that studying a different set of genes makes for a quicker and much more efficient heart disease test.
The new test, currently only available in the UK, makes it less expensive and easier to quickly find, diagnose, and move on to treatment of any type of inherited heart condition. The British Heart Foundation and the Health Innovation Challenge Fund supported this more advanced type of genetic research in order to help save the lives of the many families that share life-threatening genetic diseases that affect the heart.
Brand Beat: Advertised Drugs are Prescribed to Many Patients Who Request Them
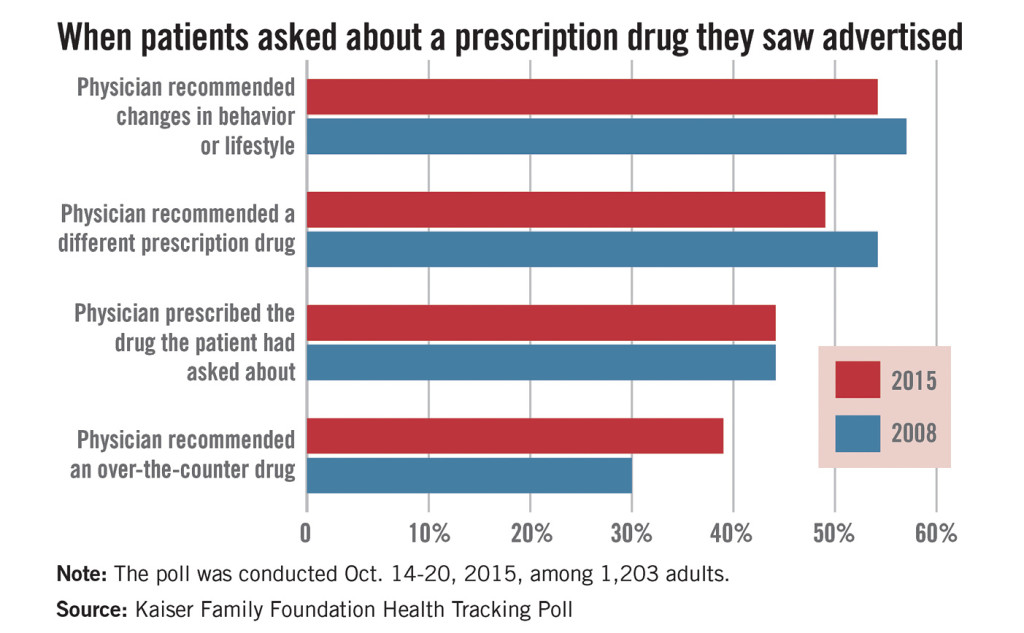 Wonder if drug advertisements lead to patient requests for your brand? According to a 2015 Kaiser Family Foundation Health Tracking Poll, many do—and they get them. The poll, based on phone interviews with 1,203 U.S. patients, found that 28% of patients request advertised brands, and of that number, 44% get them, which means drug advertising is paying off.
Wonder if drug advertisements lead to patient requests for your brand? According to a 2015 Kaiser Family Foundation Health Tracking Poll, many do—and they get them. The poll, based on phone interviews with 1,203 U.S. patients, found that 28% of patients request advertised brands, and of that number, 44% get them, which means drug advertising is paying off.
However, in lieu of brand drugs, physicians also recommended lifestyle/behavior changes (54%), prescribed a different prescription drug (49%), or prescribed an OTC drug (39%). The results of a 2008 Health Tracking Poll showed fairly similar responses.
Therapeutic Talk: Can Algae Restore Sight?
Surgeons will attempt to help a legally blind person see with a procedure that involves injecting a virus filled with DNA from algae directly into the eye. RetroSense Therapeutics has sponsored the first photogenetic study, combining gene therapy to control nerve cells’ response to light. The Retina Foundation of the Southwest will actually carry out the procedure on 15 patients who have slowly gone blind through a condition called retinitis pigmentosa and cannot see much more than a hand waving inches from their face.
Most blindness occurs due to a loss of photoreceptor cells, which receive light and transmit it to the brain through nerve cells. The injected light-sensitive algae DNA will program the ganglion cells to transmit a response to nerve cells instead. The researchers expect this to produce substantial vision, but because the algae only respond to the blue component of light, the patients’ vision may be black and white.
FDA Update
Drug Approvals
Aurobindo Pharma received FDA approval to make and market 150 mg ibandronate sodium tablets. The tablets are used to prevent bone loss, slow bone loss, and rebuild bone mass in postmenopausal women with osteoporosis. The drug is bioequivalent and therapeutically equivalent to Hoffman-La Roche’s Boniva.
Evomela, made by Spectrum Pharmaceuticals, received approval for two indications: The use as a high-dose conditioning treatment prior to hematopoietic progenitor (stem) cell transplantation (ASCT) in patients with multiple myeloma (MM) and the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Expanded Approvals
The FDA has approved Pfizer’s Xalkori capsules for ROS1-positive lung cancer tumors. Testing proved the drug efficient in response rate and the duration of response when patients were given 250 mg doses twice daily. Patients with this rare form of the disease make up approximately 1% of U.S. patients with non-small cell lung cancer.
Breakthrough Designation
The FDA granted this designation to Novimmune for its NI-0501 treatment for patients with primary HLH with refractory disease. The treatment is the first targeted therapy for the disease.
Med Device Approvals
The FDA granted marketing approval of a disposable contact lens that informs optometrists as to the best time of day to measure IOP, elevated levels of which indicate nerve damage associated with glaucoma. Sensimed AG’s Triggerfish senses changes in eye volume and transmits the data wirelessly over a 24-hour period. Approval was granted after multiple studies proved Triggerfish as effective and tolerable for the wearer.




