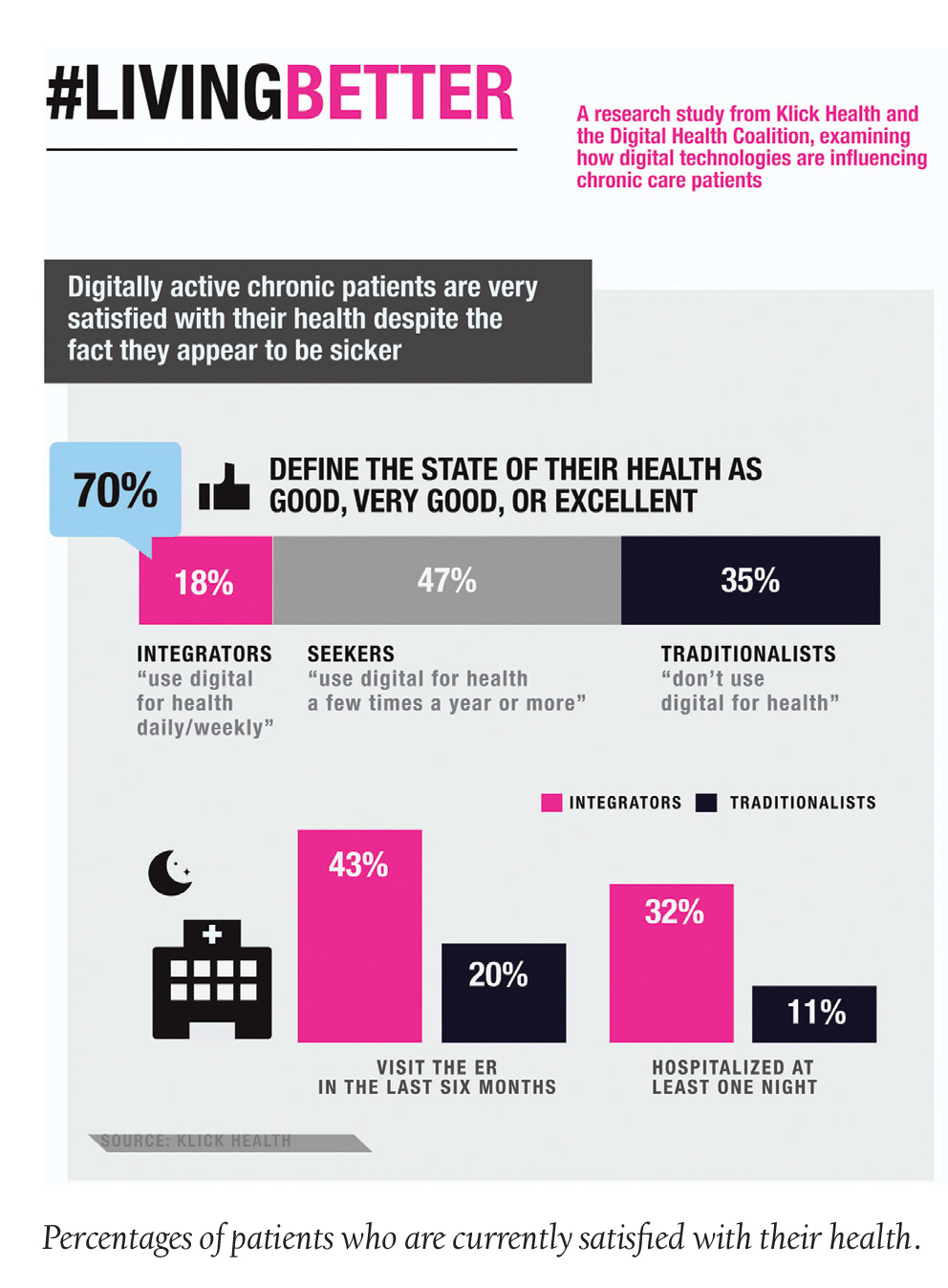
TeleMed Texts: Health Tech Help Chronic Condition Patients Feel Healthier
A recent study conducted by Klick Health, with help from Google and Digital Health Coalition, suggests that chronic condition patients are more satisfied with their health when using digital health technologies, even though they may be less healthy than those who do not use technology management. Klick’s #LivingBetter study found that 18% of patients with chronic conditions use web-based health management systems, such as WebMD, and 70% of these patients feel they are in good to excellent health—despite the fact that 43% have visited an emergency room and 32% were hospitalized for a night in the past six months.
Chronic patients who do not conduct healthcare activity on the web said the same things about their health, but only 20% went to the ER and only 11% had been hospitalized at all. “Health tech is enabling patients to proactively manage their conditions, which could be impacting their positive outlooks,” says Peter Flaschner, SVP, Client Experience. Klick’s study also shows that of those patients who use the web for health-related activity, most would like to see more patient-to-physician interaction via digital devices, such as apps.
Discoveries/Innovations: Researchers Make Headway on Herpes Vaccine
For nearly three decades scientists have been trying to develop a herpes vaccine using a single protein that is known for its robust production of antibodies and is located on the virus’s outer surface. Maybe they should have been trying the exact opposite approach.
Howard Hughes Medical Institute (HHMI) scientists at Albert Einstein College of Medicine have developed a promising vaccine for herpes by creating a genetic mutant lacking that protein. The vaccine works by inducing antibody-dependent cell-mediated cytotoxicity (ADCC), a process in which antibodies attach to a virus and then flag it for the immune system to destroy. In studies, lab mice have been completely immunized against genital ulcers, a form of herpes, using the vaccine.
“With herpes sores you continually get them,” William Jacobs, an HHMI investigator at the Albert Einstein College of Medicine, said in a statement. “If our vaccine works in humans as it does in mice, administering it early in life could completely eliminate herpes latency.”
Patient Pages: Seniors Want Healthcare Via Technology
In the coming years, more funding for healthcare technology will be geared toward providing seniors with at-home access to healthcare. Studies released by Accenture note the need for these technologies in findings revealing that 67% of seniors want to access their healthcare via technology while more than three-fifths would like wearable health monitoring devices. In fact, AARP estimates that $266 million will go into funding for heart rate and blood pressure trackers alone.
As they grow more tech savvy, this group also wants to access healthcare communities online to feel more secure about their doctor’s recommendations, and 25% want to access their medical records—such as lab results—online. Demand is also growing for access to a variety of healthcare technology—including virtual physician consultations. However, very few physicians currently offer these types of services. Accenture also indicates that a majority of U.S. government officials believe technology could be key to alleviating challenges of healthcare for the increasingly older population.
Med Device: Better Walk to Release a Crutch Alternative
 A medical device company that develops solutions for musculoskeletal diseases, Better Walk, is now developing a crutch that enhances the mobility and improves the experience of those who rely on crutches. Partha Unnava, CEO of Better Walk, was inspired to improve crutches after experiencing firsthand the pain they cause in addition to the original injury.
A medical device company that develops solutions for musculoskeletal diseases, Better Walk, is now developing a crutch that enhances the mobility and improves the experience of those who rely on crutches. Partha Unnava, CEO of Better Walk, was inspired to improve crutches after experiencing firsthand the pain they cause in addition to the original injury.
So that Better Walk can continue to develop new mobility devices for patients with ongoing needs, the company chose MB Venture Partners, a capital firm based in Memphis, to lead their funding. In March, the company closed a Series A round of funding at $450,000 and, at press time, their total funding has now reached $600,000. Unnava said in a statement, “The funding will help us to continue down the path of manufacturing and distributing the Better Walk crutch to patients who need it.”
Doctor Docs: Prescription Drug Monitoring Programs Need Improvement
Prescription Drug Monitoring Programs are state-run databases that track federally controlled substance prescriptions for the sake of targeting drug abuse. The databases allow physicians to identify patients who have prescriptions from multiple doctors before they prescribe a controlled substance, such as opioids.
While about 72% of 420 physicians are aware of the program, only 53% actually use it, according to a Johns Hopkins Bloomberg School of Public Health research study. This leaves a large amount of physicians who are unaware of or do not use the program. And the physicians who do use it think some improvements are in order: 28% of respondents indicated that the information was not in an easy-to-use format. Meanwhile, 58% reported that the info was just too time-consuming to retrieve.
FDA Update
Drug Approvals
Fresenius Kabi USA received approval for Argatroban Injection, a type of blood thinner. The drug is used to prevent blood clots that cause heart attack or stroke. The injection is also used during procedures to open blood vessels.
Sanofi Pasteur’s Quadracel received approval for active immunization against diphtheria, tetanus, pertussis and poliomyelitis in children between the ages of four and six.
The FDA recently approved Emergent BioSolutions Inc.’s Anthrasil for the prevention of inhalational anthrax. This approval triggers a $7 million payment to the company under a development contract with the Biomedical Advanced Research and Development Authority (BARDA). Anthrasil was developed as part of a $160 million contract with BARDA, within the office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health and Human Services.
Device Approvals
A new heart pump from Abiomed, Inc. received approval from the FDA. The Impella 2.5 System is a miniature blood pump intended to help certain patients maintain stable heart function and circulation during certain high-risk percutaneous coronary intervention (HRPCI) procedures, such as balloon angioplasty and stenting.
Kaneka Pharma America’s Lixelle Beta 2-microglobulin Apheresis Column is the first-ever device approved to treat dialysis-related amyloidosis (DRA).
The FDA expanded the approved use of Medtronic’s CoreValve System to include valve-in-valve procedures in patients whose surgical aortic heart valves have failed. This is the first-ever device approved for this kind of procedure.