Discoveries/Innovations: Blood Test Predicts Response to Cancer Meds
New progress was made at the Karolinska Institute and University Hospital in Sweden. Investigators have shown that a simple blood test, called DiviTum, can predict which groups of breast cancer patients would respond best to treatment, how long they would be treated and their survival rate. Manufactured by Swedish bio-diagnostic company Biovica, DiviTum analyses cell division rate in the blood. In the “TEX” study, 287 women with breast tumors who were treated with two different kinds of chemotherapy were compared and tested with DiviTum. A total of 53% of women in the study responded to either treatment, and tumors in 11 breast cancer patients disappeared. Analyses of cell growth rate revealed that patients who had a low DiviTum value lived 17 months longer, at 38 months, compared to those with higher values, at 21 months. The results of the study were presented in early May at the IMPAKT scientific conference in Brussels.
Patient Pages: PMI Initiative Well Received by Patients
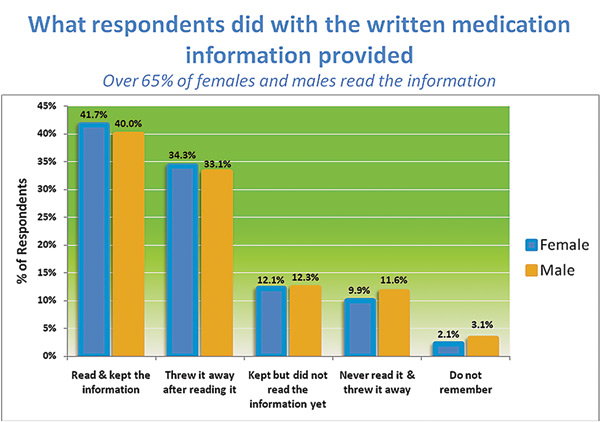
Catalina Health and other partnering healthcare organizations spearheaded an initiative called the Patient Information Quality Improvement (QI) Initiative in the summer of 2012 that disseminated newly designed patient medication information (PMI) to patients filling prescriptions at participating pharmacies to ensure better comprehension and adherence. After eight weeks of study, the initiative garnered very positive feedback. The QI initiative surveyed patients online and by phone to confirm whether or not they received PMI with their prescriptions, assessed whether or not they found the information useful, and determined how they would like to receive this newly formatted patient medication information in the future. The study showed that males and females and all age groups from 18 to 65+ had a 90% recall rate of receipt of the single-page PMI document. More than 65% of females and males read the information, and more than 90% of all patients found the PMI useful. See the chart below to find out what the patients did with the info.
Sales Sector: Physicians Not Receiving Side Effect Info
That’s right. According to an international study of physicians, family physicians were not receiving information on harmful side effects from pharma sales reps. These physicians were also prescribing these drugs, despite the lack of side effect info. That’s a big uh-oh.
The report titled “Pharmaceutical Sales Representatives and Patient Safety: A Comparative Prospective Study of Information Quality in Canada, France and the United States” was published online in the Journal of General Internal Medicine.
The study surveyed 255 physicians who completed questionnaires following each encounter with pharma sales reps in order to gauge how often information was provided about a drug’s safety profile. Researchers found that sales reps failed to provide information on common or serious side effects for drugs they promoted in 59% of physician office visits. Of 1,692 drug promotions physicians reported, only 6% of the drugs promoted had serious adverse events reported. Interestingly, although France does not have “black box” label regulations, physicians in France were more likely to be told of a harmful effect in a promotional visit, compared to physicians in the U.S. and Canada, which do have the warning labels.
Therapeutic Talk: Most Used First-Line Treatment for Prostate Cancer
In spite of its recent FDA approval in December 2012, Janssen Pharma’s late-stage prostate cancer treatment, Zytiga, has become the most used first-line treatment for the disease, according to new findings from Kantar Health’s oncology report, CancerMPact Monthly Drugs and Regimens. The report revealed that Zytiga’s market share rose to 30% since its launch in 2011. Within a month of the FDA’s approval, its market share increased to 43% as a first-line therapy, while its competitor’s share, Sanofi’s Taxotere, dropped from 75% to 40% by that time. The report also showed that Zytiga is the preferred second-line treatment of metastatic prostate cancer, with a nearly 60% market share, and has a 30% market share as a third-line therapy. According to Kantar Health’s Vice President, David Robinson, Zytiga performed so well as a first- and second-line treatment because of its favorable safety profile and high level of efficacy. He also attributed the success to physicians wanting a “non-chemotherapy option like Zytiga because they prefer to withhold chemotherapy until patients are symptomatic to spare them the toxicity that comes along with chemotherapy.”
Doctor Docs: Communicating with NPPs
The results of an interesting new study, “The Non-Prescribing Physician Will See You Now” conducted by GSW, will have healthcare marketers re-thinking their strategies to non-prescribing physicians (NPPs). Physician assistants and nurse practitioners are not the only NPPs. As revealed by this study, clinical nurse specialists, certified nurse midwives and certified nurse anesthetists represent a growing population of healthcare treatment decision makers.
The study, which details the driving forces behind the prescribing habits of NPPs, surveyed approximately 400 NPPs in 46 states from outpatient clinics, private practices, nonprofit clinics, private outpatient clinics, urgent care facilities and retail healthcare establishments. In addition, the study also looked at ways in which NPPs connect with each other and how they stay informed of the most up-to-date medical information, and how the pharmaceutical and medical device industries can best collaborate with this audience.
The study revealed that 1) 70% of NPPs connect with their peers at in-person national conferences or professional meetings, 51% online and 48% over the phone; 2) 98.8% of NPPs consider medical journals as a primary resource for medical information; 3) 94% of NPPs use online peer communities; and 4) 74% of NPPs use electronic medical records.
FDA Update
Request to Stop Pain Generic Blocked
Actavis and Impax Labs can continue to make generic versions of Opana ER. Endo Pharma, the maker of Opana ER, requested that the FDA withdraw the generic versions on the basis of their claim that the product is not tamper resistant and is unsafe. The redesigned formulation of Opana ER featured an anti-crush design meant to deter abusers. However, based on studies, the FDA found that the newer version launched in 2012 did not slow abuse and can still be tampered with by abusers.
Biosimilars Update
Florida joins six other states, including most recently Indiana, in declining the biosimilar legislation that would create barriers for patients to access newer, lower-cost versions of biologic medicines. The Florida bill contained provisions which were problematic such as limitations on substitution, additional paperwork requirements and mandates for notifying physicians beyond current law. The Florida legislature voted to approve a bill that follows current substitution practices for prescription medicines. An approval of this law would reject efforts of Amgen and Genentech to limit access to these medicines once they reach the market.
Recent Approvals
GlaxoSmithKline and Theravance obtained FDA approval to market Breo, a combination of the new beta agonist vilanterol and fluticasone furoate, a corticosteroid, as a treatment for chronic obstructive pulmonary disease.
Novartis’ Ilaris (canakinumab) was approved to treat a serious form of childhood arthritis. Ilaris is an interleukin-1 beta inhibitor intended to be administered as a monthly subcutaneous injection.
Ohio-based X-Spine received clearance from the FDA to launch its medical device, Zygafix Facet Fusion System for fusion and stabilization of the facet joint. The device uses a hollow fenestrated titanium compression screw with an internal bone graft and can be implanted through minimally invasive techniques.