Discoveries/Innovations: Electronic Tattoos to Track Your Health?
Researchers have come up with a way to “print” devices onto the skin for a period of time to track health and monitor healing of skin wounds. That’s right—electronic tattoos. Made up of ultrathin electrodes, electronics, sensors, and wireless power and communication systems, these devices can be attached to the skin and record and transmit electrophysiological measurements for medical purposes. The devices were tested at the University of Illinois at Urbana-Champaign and researchers found out how to print the ultrathin mesh electronic device right onto the skin. Head researcher, John Rogers, discovered that the electronic tattoo can be applied to the skin using a rubber stamp and “spray-on bandage” products to add a thin protective layer to bond the device to the skin.
The device can be worn for up to two weeks and can measure temperature, strain, and the hydration state of the skin. Rogers is now focusing on refining and developing communication systems for the device and believes his company, MC10 Inc., can launch these electronic tattoos.
Trend Setting: Study Reveals MS Patients More Educated
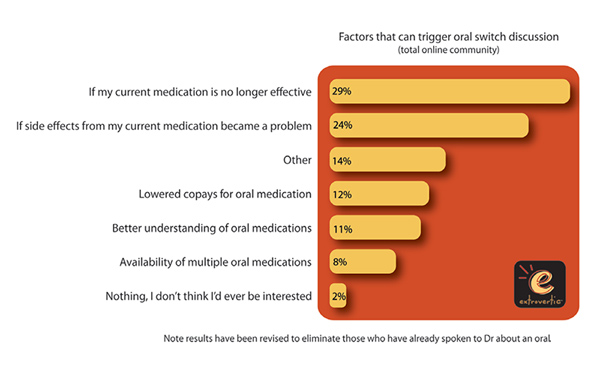
According to Extrovertic’s Patient Engagement Study: Eye on MS, the first in a series of studies looking at online patient community engagement, MS patients are engaged, very positive, well-informed and ready to act. They have an insatiable appetite for information about MS medications and a strong interest in the new orals; they are very open to talking about their MS.
The study showed that 61% of the online patient community is ready to ask their MS doctor about switching to an oral medication (from an injectable), if they are assured that the new options are as effective as their current medications. The study also showed the popularity that online communities have among MS patients. Most active patients contribute to online community discussions more than once a week; 47% participate in six or more online MS communities; 62% of these communities have over 1,000 members, while 16% include over 10,000.
In light of this trend, Extrovertic’s founding partner, Dorothy Wetzel, advises brands to approach marketing and patient outreach differently: Provide patients with tools and messages to help them talk about their MS with friends, family and the general public, and prepare MS patients for product-switch conversations with their specialists.
DC Dispatch: Battle for Biosimilar Law Acceptance
Proposed substitutions of FDA-licensed biological products for biosimilars, a pathway put in place by the Biologics Price Competition and Innovation Act (BPCI Act) within President Obama’s Affordable Care Act is growing more unfavorable state by state. Maryland, the latest state to consider this law declined to move forward with the legislation.
Maryland is the fifth state that has declined to pass legislation. The debate among legislatures centers around whether to include requirements for physicians and pharmacists on biological substitution and whether dispensers may substitute one biologic product when a different biologic product is prescribed.
Biotechnology Industry Organization (BIO) issued its own framework for states to follow in adopting a policy on biological substitution. The BIO framework advises substitution only when the FDA has designated a biologic product as a “biosimilar;” authorizes prescribing physicians to prevent substitution; requires both the prescribing physician and patient to be notified of substitution; and requires pharmacists and the physicians to keep records of the substitution. The FDA’s proposed guidelines relating to biosimilars, on the other hand, have not yet been finalized. Until the FDA issues final guidance on biosimilars, declining states call the legislation “premature” and “unnecessary.”
Therapeutic Talk: Gastrointestinal Devices Poised for Growth
Reports by business information provider, Visiongain, forecast that the gastrointestinal devices market will reach $18.2B by 2015 and will grow steadily to 2023. Increasing demand for safer and cost-effective therapies is positioning these devices for significant potential for growth and investment.
The gastrointestinal market is a highly competitive market, but highly attractive and profitable. The market is dominated by a few large manufacturers, but several small manufacturers that offer innovative and niche products are poised for growth. Currently, there are gastrointestinal devices for the treatment of a wide range of disorders including oesophageal cancer, stomach cancer, colon cancer and Crohn’s disease.
Visiongain research suggests that new products and line extensions of existing products will be important for rate of growth. Visiongain’s healthcare industry analysts predict that the gastrointestinal endoscopy market in particular will see growth due to a positive reimbursement outlook.
TeleMed Text: “Celebrate Someone” App
Imagine being able to celebrate someone who goes the extra mile using an app. Cool, right? Well pharma company Sanofi US created an app to say “thank you” to someone who has made a difference. They recently launched a Severe Allergy Awareness Facebook page where followers can give an award to an individual who (or a group, company or organization that) has helped them or a loved one avoid a life-threatening allergic reaction or manage their severe allergies via the “Celebrate Someone” app.
For the first award someone makes, Sanofi US will donate $5 to a patient advocacy group focused on severe allergies—up to $100,000 total. The donations will benefit Food Allergy Research & Education (FARE), Kids with Food Allergies Foundation (KFA) and the Asthma and Allergy Foundation of America (AAFA).
Followers can visit www.facebook.com/severeallergyawareness and the “Celebrate Someone” app to create an award.
FDA Update
Budget Request for Product Safety
Despite expectations of a cut to the FDA’s food safety budget triggered by the sequester, the FDA requested a budget of $4.7 billion to protect and promote public health as part of President Obama’s fiscal year (FY) 2014 budget. Industry fees would fund 94% of the budget increase, including new fees to support the landmark Food Safety Modernization Act (FSMA) and strengthen the FDA’s ability to oversee imported food. The remainder of the budget increases would support programs that are necessary to preserve the safety of medical products and meet the agency’s growing duties.
Consumer Update
In a recent consumer update, the FDA discussed what generic drugs are and how the FDA ensures the safety and efficacy of alternatives to brands. This update was written as a response to new complaints or evidence indicating that a generic drug may not have the same safety or efficacy as the branded product. The FDA explains and reassures consumers that in order to approve a generic drug, the manufacturer must show that the generic is equivalent in ingredients, dosage, strength and other factors to its brand-name version.
Recent Approvals
The FDA approved Novartis’ TOBI Podhaler, a plastic, handheld inhaler device that contains tobramycin inhalation powder for the management of cystic fibrosis patients with Pseudomonas aeruginosa, a bacterium that causes lung infections. Cystic fibrosis causes the body to produce thick, sticky mucus that builds up in the lungs and blocks airways, and can attract bacteria like P. aeruginosa to grow and cause chronic lung infection.
Biogen Idec’s Tecfidera (dimethyl fumarate) was approved to treat adults with relapsing forms of multiple sclerosis (MS). Two clinical studies showed that Tecfidera-treated patients had fewer MS relapses than people taking a placebo. One trial showed that those taking the new drug experienced a worsening neurological disability less often than patients taking a placebo.