TeleMed Text:
Majority of Patients Find Health Info on Internet
Doctors may be unaware of it, but patients search the Internet for health info all the time. A recent Pew study, in fact, found that 59% of U.S. adults look online for this info.
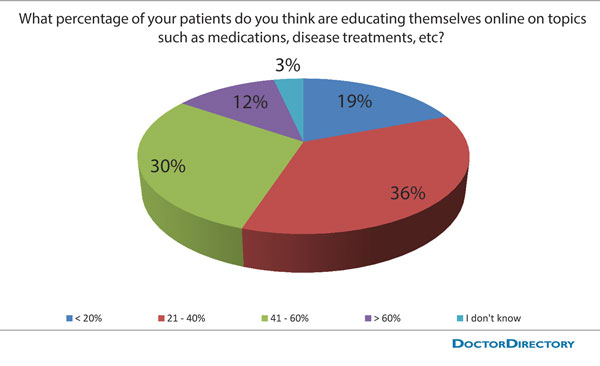
But a recent DoctorsDirectory survey of 300 doctors found that they believe this number to be much lower. Results of the survey revealed that 19% of doctors think that less than one in five patients look online. Another 36% believe that about three or four out of 10 patients search the Internet. Only 30% guessed correctly at 40% to 60%.
For more information about this survey, visit contactdd.com.
Doctor Docs:
Gifts to Medical Students are Becoming a No-No
Policies restricting the gifts that drug makers can give to medical students may have lasting effects, according to a study recently published in the British Medical Journal. And most U.S. schools now have instituted policies that restrict or ban gifts altogether.
Pointedly, researchers found that doctors who attended U.S. medical schools with these types of restrictions were less likely to prescribe two new brand-name drugs: ADHD drug, Vyvanse, and antipsychotic, Invega. Instead, they prescribed similarly effective, but older and cheaper drugs. Based on findings, researchers suggest that the policies restricting gifts to medical students can affect what they prescribe when they become doctors—although the research doesn’t prove this.
According to Dr. Joseph Ross, associate professor of medicine at Yale University and co-author of the study, banning gifts is a very good thing. He notes that gift-giving allows a certain amount of influence. Ross says it creates a relationship between pharmaceutical manufacturers and doctors that can lead to unnecessary prescriptions for newer, pricier, but not necessarily more effective drugs.
Ross adds that he believes the pharmaceutical industry has no place in educating doctors. In terms of new drugs coming to market, Ross says patients will be eager to use drugs that really make a difference.
DC Dispatch:
Final Rule on Sunshine Act Published
Financial transparency is an issue the American Medical Association (AMA) has long supported. In fact, a recent AMA report states that the organization believes that doctor relationships with industry should always be transparent, meaningfully independent, and focused on benefits to patients. It may not be so now, but according to the final rule of the Sunshine Act, published by the Centers for Medicare and Medicaid Services (CMS) February 1, 2013, it will be—soon.
Passed in 2010 as part of the Patient Protection Affordable Care Act, the long awaited Sunshine Act makes it a requirement for applicable manufacturers of covered drugs, devices, biologics and medical supplies to report financial arrangements made with doctors and applicable healthcare providers, specifically training hospitals. Manufacturers include those whose products are covered under the Children’s Health Insurance Program (CHIP), Medicaid or Medicare. The rule also requires certain manufacturers and group purchasing organizations (GPOs) to disclose physician ownership or investment interests.
The idea behind the rule is two-fold: to provide transparency to patients in the hopes that they can make better, more informed healthcare decisions; and to help with conflicts of interest that physicians or teaching hospitals can face due to their relationships with manufacturers.
The first of the Sunshine Act’s new annual reports is due at CMS no later than March 31, 2014. It will cover the period from August 1, 2013 to December 2013. CMS will publish these reports on a public, searchable website.
To download the final rule, go to bit.ly/SSARule, or bit.ly/AMARule.
Sales Sector:
Top Five Drug Gains and Losses
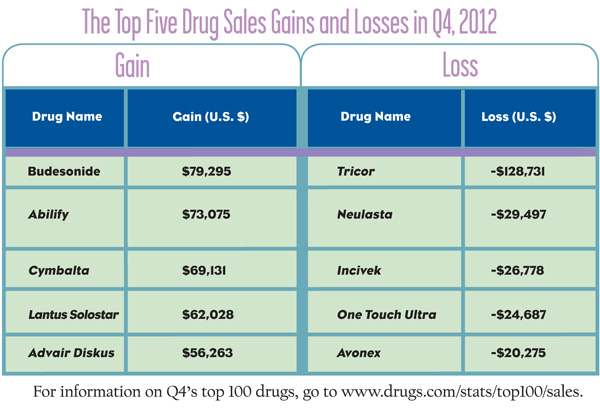
Quarter four (Q4) earnings are out—and there may be a few surprises. The top five sales gains in Q4 included generic budesonide, used to treat asthma, non-infectious rhinitis and Crohn’s disease, with a 42.52% rise in sales over Q4. But, in an unforeseen rout, Otsuka’s antipsychotic, Abilify, overtook AstraZeneca’s acid reducer Nexium to claim the number one spot of the top 100 drugs with a gain of 5.20% in Q4. Abilify is followed by Eli Lilly & Company’s Cymbalta, which came in with a gain of 5.97%; Sanofi Aventis’ diabetes drug, Lantus Solostar is up 11.58%; and GlaxoSmithKline’s COPD treatment Advair Diskus saw a 4.90% increase in its sales.
Meanwhile, the biggest losses in revenue are headed by AbbVie’s cholesterol treatment Tricor, dropping 53 spots and representing a 41.52% loss of sales; Amgen’s chemo drug, Neulasta dropped by 3.54%; Vertex Pharmaceutical’s hepatitis treatment, Incivek, fell 9.14%; and Lifescan Inc.’s glucose meter, One Touch Ultra, reported a loss of 6.59%, while Biogen’s MS treatment, Avonex, dropped in sales by 5.17%.
According to Philip Thornton, CEO of Drugs.com, fewer blockbuster drugs lost exclusivity in Q4 of 2012, but 2013 will hold challenges for some drug manufacturers. Eli Lilly & Company’s Cymbalta is expected to lose its patent later this year, as are top sellers AstraZeneca’s migraine medication, Zomig, and Eisai Inc. and Jannsen Pharmaceutical’s acid reflux treatment, Aciphex.
For information on Q4’s top 100 drugs, go to www.drugs.com/stats/top100/sales.
FDA Update
New Guidance on Alzheimer’s disease
The FDA released new draft guidance titled, “Guidance for Industry, Alzheimer’s Disease: Developing Drugs for the Treatment of Early Stage Disease,” that is designed to assist developers of new treatments for patients in the early stages of Alzheimer’s disease. The proposal explains the FDA’s current thinking about the way researchers can identify and select patients with early Alzheimer’s disease, or those who are at risk of developing the disease, for participation in clinical trials. To read the full proposal, visit bit.ly/FDAGuide.
Takeda’s Diabetes Trio
The FDA approved three new related products from Takeda Pharmaceuticals America for use with diet and exercise to improve blood sugar control in adults with type 2 diabetes: Nesina (alogliptin) tablets, Kazano (alogliptin and metformin hydrochloride) tablets, and Oseni (alogliptin and pioglitazone) tablets. Nesina, Kazano and Oseni were studied as stand-alone therapies (monotherapies) and in combination with other type 2 diabetes therapies, including sulfonylureas and insulin.
Approval Roundup
Pfizer’s Prevnar 13 approval was broadened to include the prevention of infections caused by the pneumococcal bacteria in children and adolescents aged 6 years to 17 years. Celgene’s Pomalyst (pomalidomide) was approved to treat patients with multiple myeloma whose disease progressed after being treated with at least two prior therapies, including lenalidomide and bortezomib, and whose disease did not respond to treatment and progressed within 60 days of the last treatment. The FDA approved the first generic version of the cancer drug Doxil (doxorubicin hydrochloride liposome injection) under the priority review system to help alleviate shortages of the drug. The generic is made by Sun Pharma Global FZE. Novartis’ Gleevec (imatinib) was approved for a new use: the treatment of children newly diagnosed with Philadelphia chromosome positive acute lymphoblastic leukemia.