Global electronic initiatives, especially in electronic data and document management, continue to evolve rapidly with far-reaching and major implications for the industry. Access to electronic data is critical for drug and device companies that want to optimize drug development, share invaluable information and file successful regulatory applications. To be effective, electronic data and document initiatives must be reliable, transparent and re-usable to inform decision-making and future projects.
Trends in Global Electronic Initiatives
In today’s environment of evolving science, technology and regulations, both industry and regulatory agencies must continually reassess their data and document management standards, processes and technology to be optimally positioned operationally and to comply with emerging requirements. Research and development, manufacture, quality control and product distribution must be thoroughly documented at every phase, and these records must be readily available for examination by regulatory authorities.
“Data management has always been an important part of the clinical trial process because the data being managed is usually the patient’s medical record, and it is vital to the patient’s safety and privacy that this information be accurate, according to Richard Chamberlain, President, Extended Clinical Services, LLC. “And the collection of data during a clinical trial forms the basis for the study results and reported outcomes and is foundational to an FDA submission. Chamberlain explains that, “Historically, this data was gathered in paper form by site personnel as the study progressed, and later entered by the pharmaceutical company after the study was complete.”
Many companies realized that the rapid influx of information management technology can ease the paperwork burden but the need for regulatory compliance and for accurate, unalterable records needs to be considered. The challenge for pharmaceutical companies engaged in clinical trial research is to implement an efficient and accurate electronic workflow that allows for the creation, circulation and approval of documents that comply with FDA guidelines for content and structure.
New technology is changing the way that clinical trial data is managed in two primary ways:
1. The Cloud
Historically, study teams reported from each study site and downloaded the site’s data to the sponsor, who consolidated the sets of data into one study database. This was time consuming and risky. With cloud computing, each site accesses the same version of the database and data management system eliminating download and merger of data sets. Potential data security and backup issues can be problematic in this cloud approach. Document security is vital for document management and compliance requirements.
2. EDMS
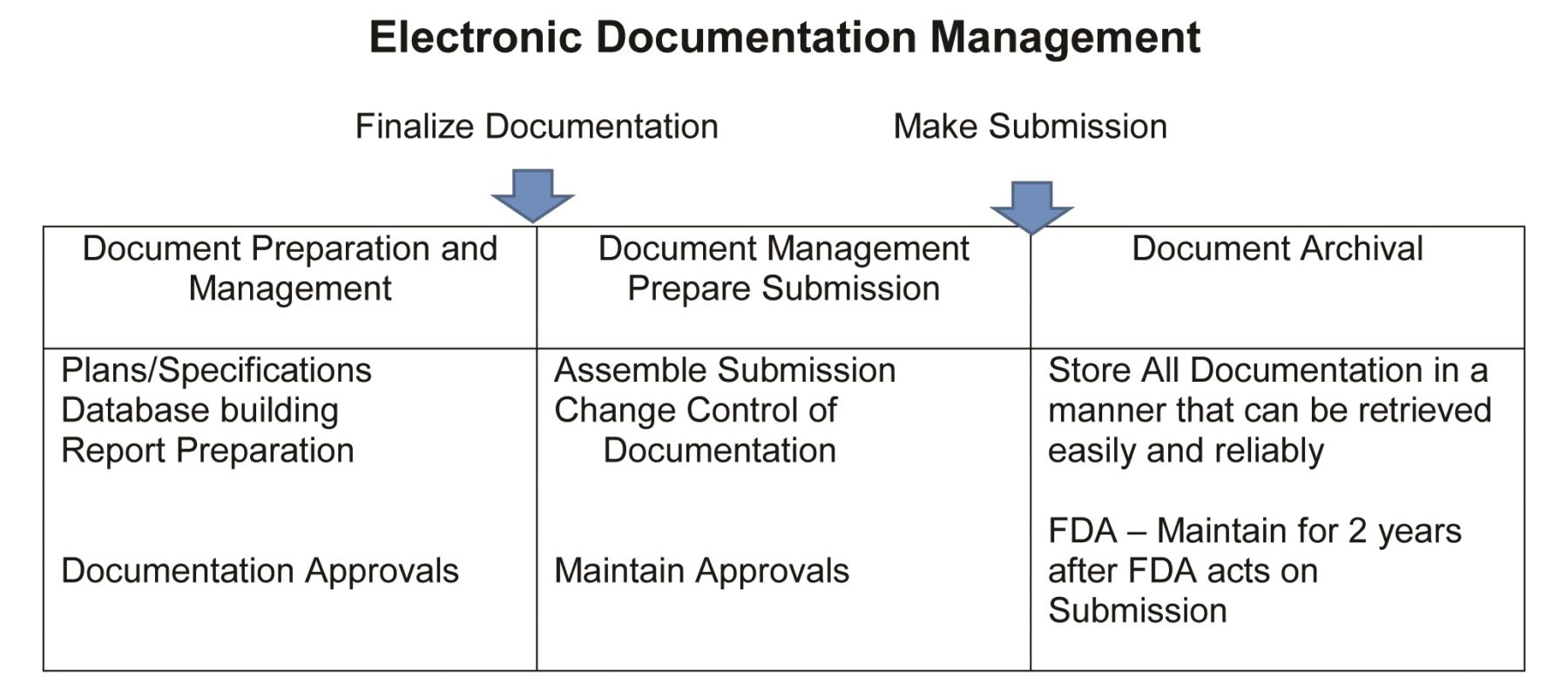
An Electronic Document Management System (EDMS) is a computer system or set of software programs that electronically manages the creation, storage and control of various document types and facilitates the movement of data within an organization’s workflow (Figure 1). Its use has major implications on the way an organization develops, archives and—specifically in the field of healthcare and drug development—delivers that critical information. It provides a means to centrally store a large volume of digital documents and often includes features that enable efficient document retrieval.
EDMS (also known as content management, records management, information management systems) should be designed for uploading, storing and retrieving information in a systematic way that meets the organization’s technical, operational and business requirements and includes the following capabilities:
- Tools to capture, upload, store, archive and control access to content.
- Monitoring of compliance and/or legal requirements.
- An indexing system.
- Search capability.
- Lifecycle management of data trail.
- Automatic routing of documents through a workflow system.
Data in healthcare organizations can often be siloed by functional departments and not integrated, making it difficult to retrieve and use in a valuable means. Developing an effective EDMS will ensure that an organization’s business, legal, operational and infrastructure needs are met. Different departmental perspectives should be considered when designing an EDMS solution so that it fits the organization’s overall culture and is responsive to the organization’s actual needs and usage.
Additional Impacts
The efficient/reliable sharing of critical information gained during product development is foundational to improved healthcare worldwide. Greater accuracy of clinical trial data, and quicker access to and broader dissemination of this information enable speedier product review and time to market—which translates to speedier delivery of the product to the patients who need it.
Risks posed by incomplete/inaccurate data could jeopardize patient safety and make needed treatments unavailable. By utilizing an EDMS, healthcare organizations increase their chances of maintaining data integrity standards.
Documents created, disseminated and organized via EDMS are the cornerstone of regulatory submissions and compliance with regulatory requirements for data are critical. An EDMS ensures that documents and their information are accurate, thorough and easily accessible.
Other major reasons that adopting an EDMS system is valuable to medical product development are listed below:
• Single data source: One integrated, indexed repository of data reduces storage needs and retrieval expense.
• Improve regulatory compliance: Compliance with records retention schedules is automated and improved; incoming documents are automatically classified and stored.
• Information reuse: Stored information can pre-populate forms or validate other information reducing the potential for missing or misplaced data.
• Increased information security: Unauthorized record access, disaster loss and data recording/transcription mistakes can be controlled.
References:
Chamberlain, RL. “Cloud Computing: New and Changing Technologies,” Global Forum 6:4, 2014.
Committee Prepares for eRegulatory & Intelligence 2015, Global Forum 6:6, 2014.
King, Gillian. “Achieving Regulatory Information Management,” Global Forum 3:4, 2011.
“Optimization Strategies for Electronic Document Management Systems,” www.stratishealth.org.
“The Basics of Electronic Document Management Systems,” www.oakleigh.co.uk.