FROM THE LANCET ONCOLOGY
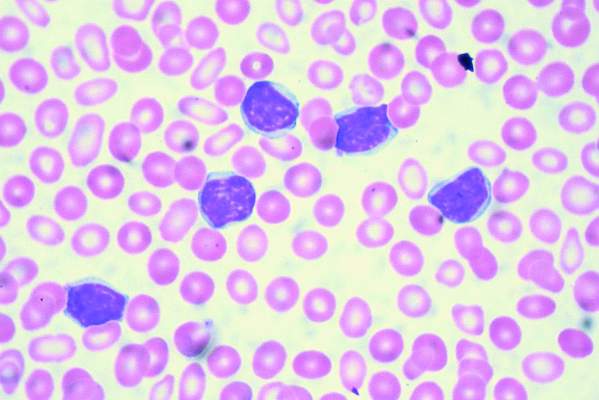
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
“Careful attention needs to be paid to management of serious adverse events and infections associated with this regimen during treatment selection,” Andrew D. Zelenetz, MD , of Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, and his colleagues wrote ( Lancet Oncol. 2017 March;18(3):297–311 ).
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
On Twitter @maryjodales