The 2018 Farm Bill redefined the meaning of hemp, opening the door to small and large companies that began producing, manufacturing, and marketing cannabinoids for a variety of purposes with little to no oversight. As federal policy lags around hemp and cannabidiol (CBD) product manufacturing and marketing, the players operating within its framework have begun taking advantage of this lack of federal oversight to the detriment of consumers.
A concerning issue is playing out across the U.S. today. Cannabis industry’s lobbyists are using observational studies and incomplete data to support arguments that cannabis-based products are safe. This situation is leaving consumers vulnerable.
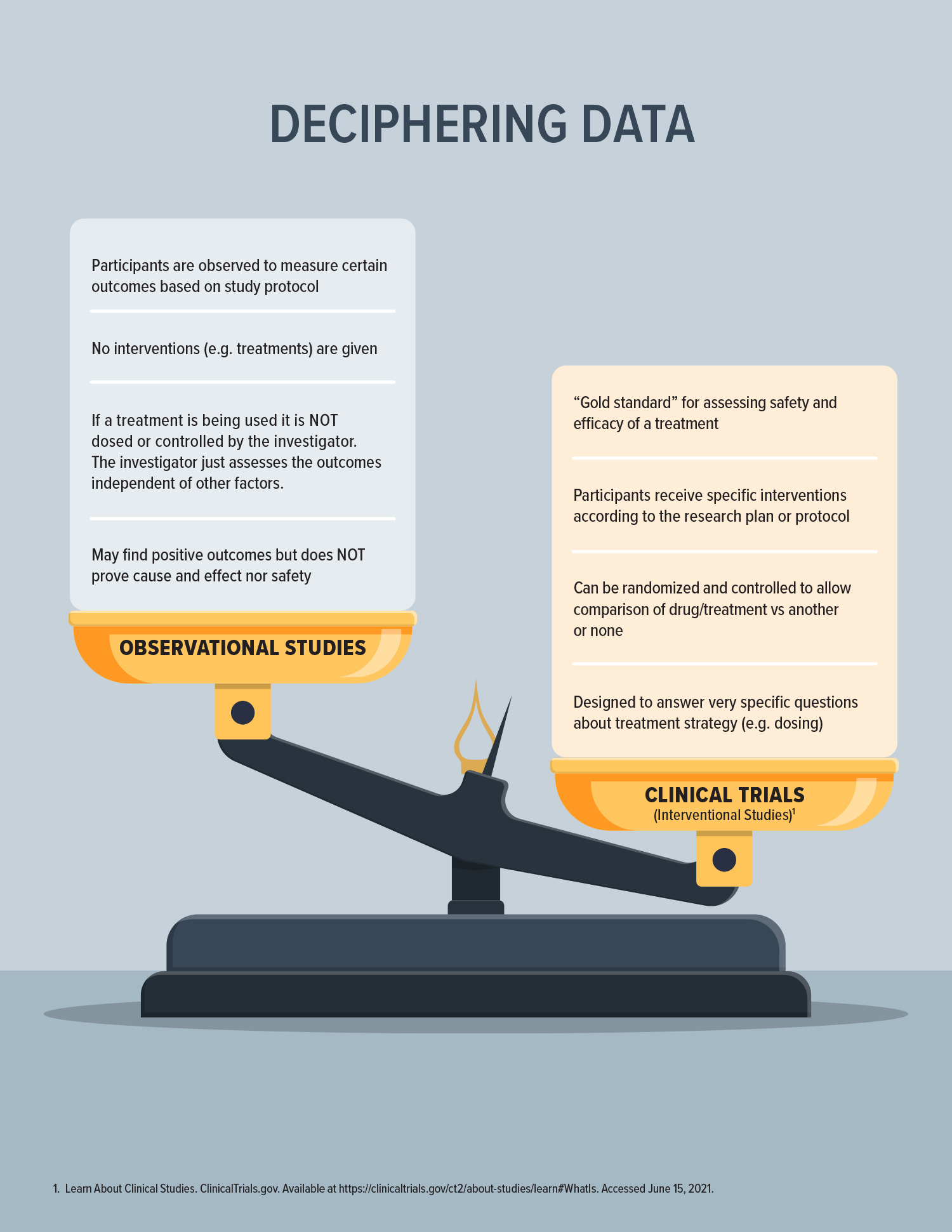
Not All Data Is Created Equal
While observational data provides valuable signals about the effects of a given drug or behavior; it does not provide healthcare providers or patients with clarity around the safety and efficacy of a drug for a specific condition at a specific dose.
In a recently released joint statement, the FDA reiterated its ongoing work to better understand the safety profiles of cannabis-based products on the market. It acknowledged the enthusiasm expressed by many groups currently providing data to the agency around these products, but countered that existing efforts “are not adequate to fill the outstanding knowledge gaps” and that “observational studies that are too small or that do not include techniques to ensure data quality or methodological rigor are of limited use for public health decision making.”1
 Without FDA approval of a cannabis product, patients and caregivers take on considerable responsibility to evaluate what they are buying, from whom, how it was grown, and whether it is safe. The FDA drug-approval process exists to protect patients by establishing the safety and efficacy profiles of prescription medicines for specific indications. The reality is most consumers aren’t aware of these nuances in laws or terminology, nor do they have the knowledge or expertise to evaluate the safety of a product, laboratory results, or the medical management of a disease.
Without FDA approval of a cannabis product, patients and caregivers take on considerable responsibility to evaluate what they are buying, from whom, how it was grown, and whether it is safe. The FDA drug-approval process exists to protect patients by establishing the safety and efficacy profiles of prescription medicines for specific indications. The reality is most consumers aren’t aware of these nuances in laws or terminology, nor do they have the knowledge or expertise to evaluate the safety of a product, laboratory results, or the medical management of a disease.
This is a reminder that even well-intentioned efforts to prove the quality of cannabis-based products need to be backed by scientific evidence. To protect the public, we must be able to decipher between good and bad data.
References:
1. “Better Data for Better Understanding of the Use and Safety Profile of Cannabidiol (CBD) Products,” FDA joint statement, available at https://www.fda.gov/news-events/fda-voices/better-data-better-understanding-use-and-safety-profile-cannabidiol-cbd-products. Accessed on June 24, 2021.









