The quest for online information nearly always starts with search engines, and Google is the world leader. Changes in its policies and methods impact everyone looking for healthcare information and need to be considered in the context of fair and balanced promotion by drug and medical device companies. So with Google’s launch of a “smarter” search engine and new ranking criteria, commercial operations and the medical, legal and regulatory (MLR) review team have new decisions to make when executing search engine optimization (SEO).
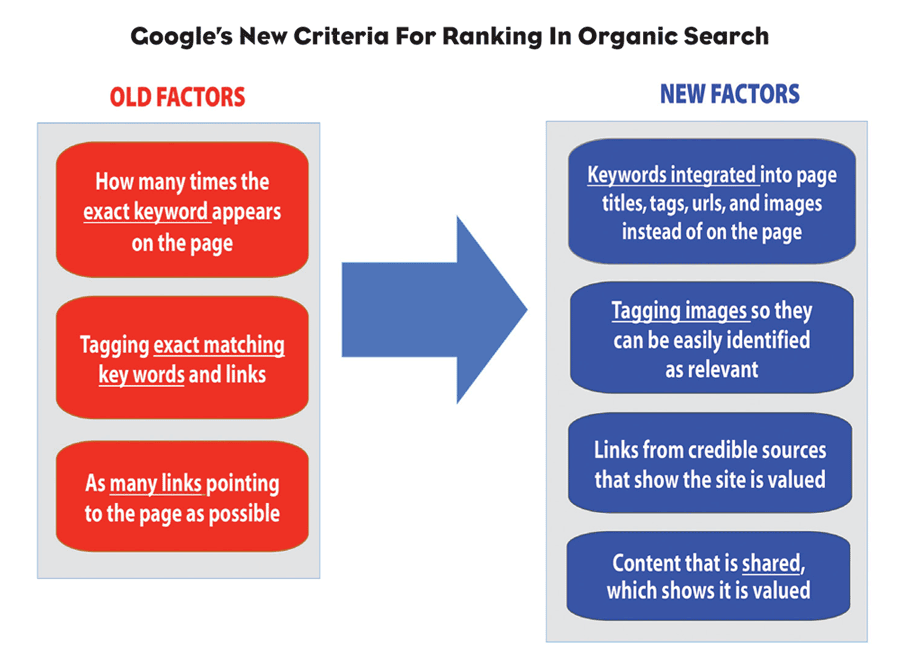
Previously, SEO was driven by keywords on the actual page viewed by physicians, patients and consumers. The more exact matching keywords, the higher the ranking in Google search results. Today, ranking is not driven by keywords on the page but by integrating keywords into the “unseen” page elements that summarize the content, such as title tags, URLs, heading tags and image codes. This raises new regulatory questions and requires marketing to rethink their SEO strategies.
Review: The Keyword’s Role in SEO
Keywords are words or phrases typed into a search engine as part of a search query. They are also used as metadata to describe images, text documents, database records and webpages. Companies specializing in SEO have access to databases that track search queries to uncover the most commonly used keywords by category or topic. They have used this information to “optimize” websites by embedding an exact match of those keywords into the page’s content and in any text links on that page. The more keywords, the more likely it was that the search engine would find the page and index it as the “answer” to a search query containing the same keyword.
Review: Organic Versus Paid Search
To ensure a prominent position on the search results page, companies buy specific keywords, for what is called a paid—or non-organic—search. Because results are colored or flagged as an advertisement or promotion, searchers can tell it’s a paid ranking. Embedding keywords in a paid search guarantees the content will be placed in a prominent position allocated for paid search on the top of the page.
Conversely, unpaid—or organic—search occurs when Google considers the site as an “authority” and assigns it a high search engine ranking. Companies do not control these rankings and even if they could embed keywords in the strategically correct places, it’s not guaranteed the site will rank for that keyword.
Organic search is more trusted and results are clicked on nine times more than paid search. However, if users are directed to a page they think is irrelevant to their search, they leave immediately, or “bounce.” The higher the bounce rate, the lower that page ranks in organic search. Once a page ranks below fourth in search results, the click-through rates average less than 5%.
A “Smarter” Google: On to Relevance
Search engines are no longer limited to matching keywords to the same keywords on a webpage. Google’s smarter search now detects content “relevance” by “reading” elements of page coding to decipher how relevant the content is to the intent of the search query—similar to Amazon’s algorithms used to suggest other books of the same genre based on relevance to the user’s current selection.
Google’s smarter search also analyzes whether too many exact matching keywords are on a page because it considers excessive use of keywords an attempt to manipulate search results. It now penalizes those sites by reducing their ranking for organic search, meaning that the common SEO practice of loading exact matching keywords will now result in a lower ranking. In fact, marketers must actually avoid exact matching of content keywords and instead tag content with keywords or parts of phrases relevant to the search query.
Regulatory Compliance and Legal Implications of an Expanded Keyword Role
This is where regulatory compliance and legal considerations enter the equation. With the new Google search, a site has a higher ranking in organic search if its keywords are relevant to both popular searches and the URL, content, title and description meta tags.
If a drug is indicated for a relatively unknown bone disease, marketers will be better served to code the URL as “bone disease types”—a more common search. However, this title tag or keyword is broader than the specific indication.
When companies broaden tags and codes, which are unseen to patients and physicians, what is a company’s oversight for that content? What happens if the broader phrases link users to information about the drug that is not on the label? Since companies directly control keyword selection and use, it’s important for reviewers to be sure that any links to external sites are within labeling and don’t broaden the indication.
These and other questions will continue to come up as Google’s “relevance” search capabilities become more sophisticated.
“It is likely that the FDA will seek to exercise oversight since the intent of keywords is to drive traffic to specific content and the FDA will want that content to be consistent with labeling—and not direct parties to misleading content,” explains Wayne Pines, a former associate commissioner of the FDA and Chair of CCC’s Advisory Board.
Putting “Smart” Search into Perspective
If marketers don’t use broader keywords and integrate them into other webpage elements, there is no guarantee their brands will be found by target audiences unless they resort to paid search. If they broaden keywords, there could be greater regulatory oversight.
Companies must define their own approach to meeting search engine criteria, and SEO experts, marketers and the regulatory reviewers must collaborate even more closely to create an effective SEO policy. Decisions need to be made in a timely fashion so physicians, patients and consumers have access to important health information more quickly than ever before.