As any pharmaceutical marketer knows, The Food, Drugs and Cosmetics Act (FD&C) requires that pharmaceutical products not be “misbranded.” The FDA has, until recently, required that pharmaceutical and biopharmaceutical companies (collectively “Life Science Companies” or “LS Companies”) avoid mislabeling their products and submit promotional content, including that added to static or interactive websites, prior to introducing the promotional content for general or healthcare provider viewership.
Due to the difficulty in engaging in an interactive medium and having to submit content prior to distribution, LS companies have avoided directly engaging patients, caregivers and healthcare providers using social media channels. The primary causes of difficulty arise from a variety of factors including the uncertainty of FDA expectations regarding the use of social media, trepidation surrounding inappropriate engagement, and the potential fines and penalties that may result from inappropriate engagements. The FDA has long claimed that social media was just another channel for engagement and implied that the rules that applied to other channels of engagement, such as television, radio and magazines, also applied to social media. However, that sentiment seemed to ignore the interactivity inherently present in social media and the acceptance of this reality seems to be reflected in recent guidances from the FDA.
Evolution of FDA Social Media Guidance
In 2009, the FDA held a public hearing on the promotion of FDA-regulated medical products using the Internet and social media tools. Speakers at the public hearing were asked to comment on seemingly unique aspects of social media including, accountability, space limitations, correcting third-party information, use of links and adverse event reporting in the context of social media. The speakers also communicated the need for the adoption of guidance measures that are Internet-specific and take into account the limitations and advances of social media technology.
After consideration, the FDA announced that it would issue guidance on various aspects of social media by the end of 2010.1 In July 2011, after no new guidance had been issued to address these needs, members of the medical information working group (MWIG), consisting of seven LS Companies, submitted a joint citizen petition requesting that the FDA clarify its policies for industry regarding four distinct types of communications that are relevant to social media and off-label discussion.2 Shortly thereafter, the FDA issued a draft guidance document, Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices. While the FDA acknowledged social media tools and technologies in the draft guidance, it focused on consumer-marketer communications.
In the summer of 2012, the Food and Drug Administration Safety and Innovation Act (FDASIA) was signed into law. This act was designed to reauthorize a number of important FDA user fee programs, promote innovation, increase stakeholder involvement and enhance the safety of the drug chain supply. Interestingly, FDASIA required that the FDA collaborate with other federal agencies to deliver, within 18 months of the FDASIA’s passage, a strategic framework for information technology regulation, and within two years of its passage, issue guidance that describes policy regarding the promotion of medical product regulated by the FDA via social media. This deadline is approaching in the next few months.3
Current State
In January 2014, the FDA finally released one of the several pending draft guidances that describe how it intends to treat social media. The draft guidance titled, Fulfilling Regulatory Requirements for Postmarketing Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics, not only provides LS Companies with the FDA’s current thinking on the submission requirements for interactive online promotional material, but also illustrates some rights and responsibilities of organizations when they engage using such mechanisms.
The FDA recommends that a company continue reporting static websites in the traditional manner, however, “interactive promotional media,” should follow the new submission recommendations in the guidance due to their real-time nature of communication.4
Submission Responsibility
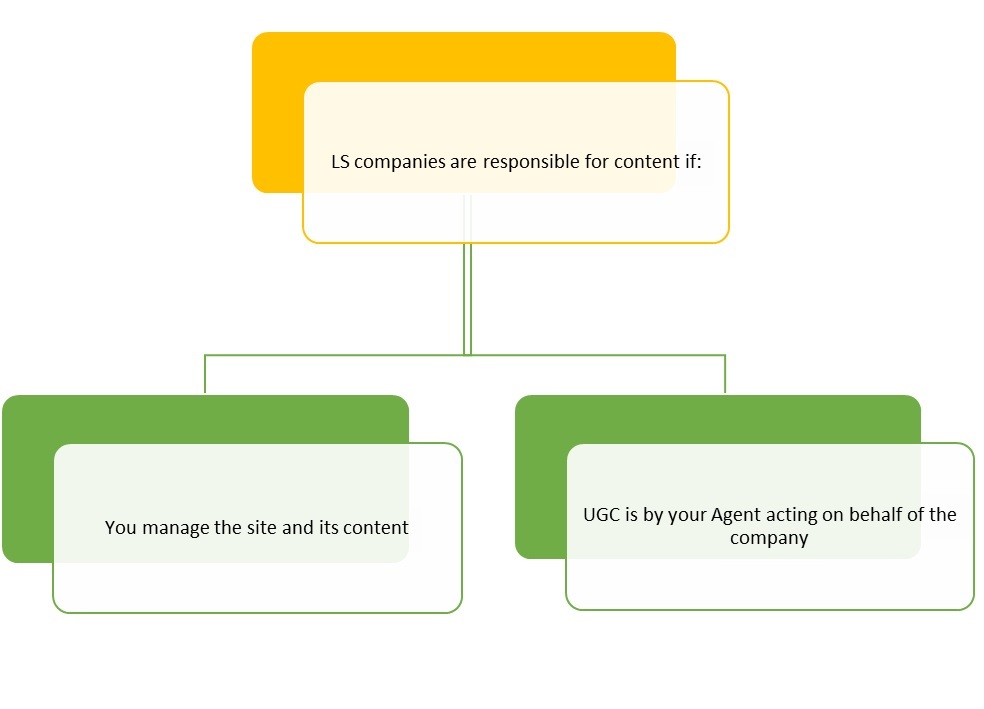
The January 2014 FDA guidance made it clearer which content a company is responsible for and what needs to be submitted to the FDA for approval. There are effectively two situations that require submission:
A LS Company is responsible for submission of static and interactive media that is Managed by the company.
A website is deemed to be Managed by a company if it is “is owned, controlled, created, influenced, or operated by or on behalf of a company.” Such Management may be exhibited in the form of control over editing, preview or review privileges to the promotional material placed on the site. Such Management may also be exhibited in the context of sites hosted on third-party servers and/or directed by third parties, such as promotional agencies, if the LS Company has any control and/or influence on the third-party site, even if it is limited in scope.
The FDA clarified that mere financial support of the site alone, without the ability to Manage the site, does not necessarily indicate control and/or influence. Additionally, if a LS Company has no editing, preview or review privileges, or does not direct the placement of the promotion within the site, the company is only responsible for submitting, to the FDA, the promotional content they provided to the site.
A company is responsible for the content generated by an employee or agent who is acting on behalf of the company.
The FDA suggests that if an employee or contractor (collectively “Agent”) of an LS Company comments on a third-party site about its product, the LS Company is responsible for the content the Agent posts on the site. Agents may not only include direct employees, but also contractors, and potentially contractors of such contractors. Therefore, a LS Company may be responsible for the User Generated Content (“UGC”) of its Agents, and such UGC may need to be submitted to the FDA.
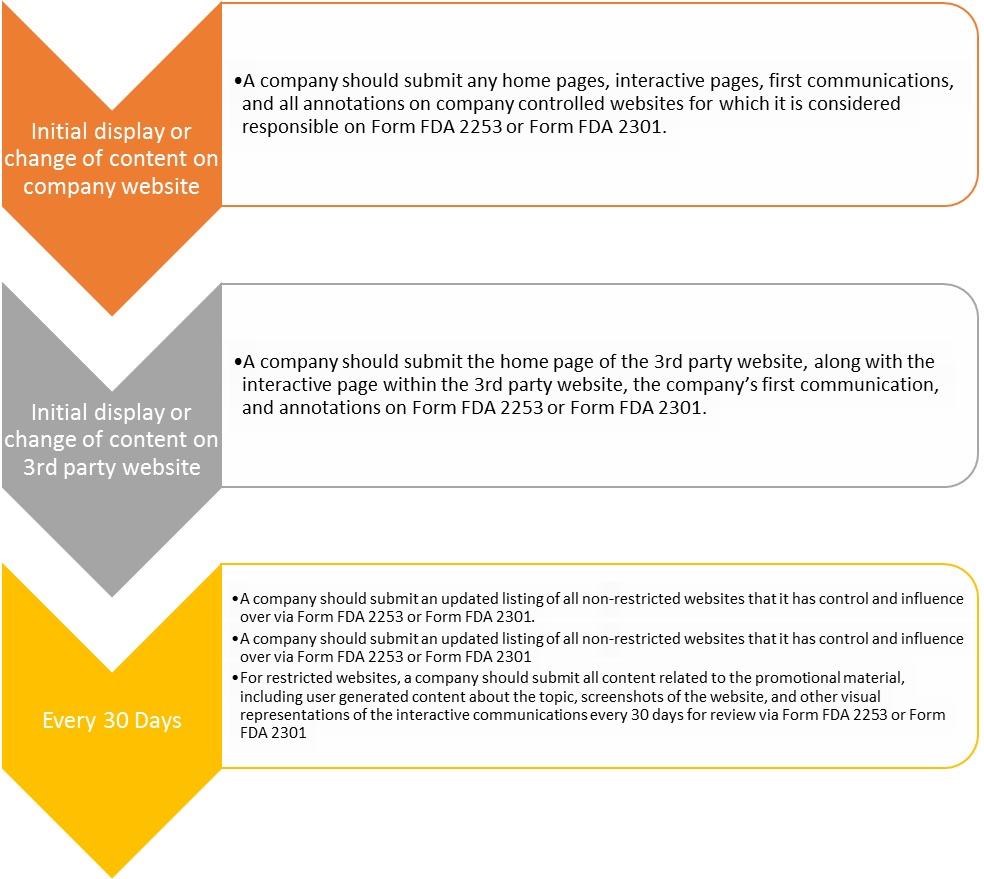
Submission Process and Timing
Initially, the FDA believed that the submission of potential promotional content to the FDA prior to exposure to the public would work for all media. However, given the interactive—and potentially real-time—nature of interactive media, such a system became untenable for LS Companies. In light of these concerns, the FDA recommended the following strategies to assist companies in their compliance efforts on websites in which they participate.
Future Guidance
Much of the industry has managed without engaging in social media for several years. In light of the impending FDASIA deadlines, LS Companies are anticipating four more guidance documents from the FDA in the coming months.5 However, as a result of various court cases from the Court of Appeals and the Supreme Court, the citizen’s petition and FDASIA requirements, LS Companies have now been emboldened to demand action from the FDA. The FDA, on the other hand, with its seemingly problematic relationship with the 1st Amendment, may be reeling with the impact of several decisions from the Second Circuit and the U.S. Supreme Court and may form a more permissive attitude towards “interactive media” media and off-label interactions.6
References:
1. The FDA agreed to release guidances on the following issues:
(A) Responding to unsolicited requests
(B) Fulfilling regulatory requirements when using tools associated with space limitations
(C) Fulfilling post-marketing submission requirements
(D) Online communications for which manufacturers, packers, or distributors are accountable
(E) Use of links on the Internet
(F) Correcting misinformation. So far, guidances on responding to unsolicited requests and fulfilling post-marketing submission requirements have been released.
Breaking – Its Official – FDA Delaying Social Media Guidance Until At Least Q1 2011, http://www.eyeonfda.com/eye_on_fda/2010/12/breaking-its-official-fda-delaying-social-media-guidance-until-at-least-q1-2011.html (Updated December 21, 2011. Accessed on March 16, 2014.)
2. The citizens petition asked for clarification on (1) Manufacturer Responses to Unsolicited Requests, (2) Scientific Exchange, (3) Interactions with Formulary Committees, Payers, and Similar Entities, and (4) Dissemination of Third Party Clinical Practice Guidelines. Reference: Citizens Petition, Dara Katcher Levy. July 11, 2011. Joint citizen petition asks FDA for clarity on off-label use manufacturer-communications. http://www.fdalawblog.net/fda_law_blog_hyman_phelps/2011/07/joint-citizen-petition-asks-fda-for-clarity-on-off-label-use-manufacturer-communications.html. Accessed March 15, 2014.
3. Will FDASIA get FDA off its butt to finally issue social media guidance? November 8, 2012. Pharma Marketing Blog website. http://pharmamkting.blogspot.com/2012/11/will-fdasia-get-fda-off-its-butt-to.html. Accessed on March 15, 2014.
4. Interactive websites were defined as “modern tools and technologies that often allow for real-time communications and interactions.” Such “tools” include blogs, microblogs, social networking websites, online communities, and live podcasts.
5. Breaking – Its Official – FDA Delaying Social Media Guidance Until At Least Q1 2011, http://www.eyeonfda.com/eye_on_fda/2010/12/breaking-its-official-fda-delaying-social-media-guidance-until-at-least-q1-2011.html (Updated December 21, 2011. Accessed on March 16, 2014.)
6. Citizens Petition, http://www.cohealthcom.org/wp-content/uploads/2013/09/citizen-petition.pdf, Last Updated September 3, 2013, Last Accessed March 16, 2014.