It is clear that Medical Science Liaisons (MSLs), and the activities they are involved with, have become vital to the success of the companies in which they work. MSLs often work throughout a product’s lifecycle. They are involved in multiple activities including ensuring that products are used effectively, serving as scientific experts to internal colleagues within companies, as well as being scientific peers and resources to Key Opinion Leaders (KOLs) within the medical community.
Although in dire need, there are no current industry guidelines that specifically address the MSL role, their training or activities. However, there are broader pharmaceutical guidelines in the U.S., which have influenced MSL activities.
In 2003, the Department of Health and Human Services Office of Inspector General (OIG) published the Compliance Program Guidance for Pharmaceutical Manufacturers, which are referred to as the OIG guidelines. These guidelines were created to address the concern that traditional commercial sales and marketing activities might influence scientific or medical activities. This impacted MSLs because prior to the guidelines being released, most MSL teams and their activities were part of marketing or sales departments. The idea behind these guidelines was to create a distinct firewall and separation between commercial and medical activities. As a result, the OIG guidelines helped to shape the future of the MSL role and their activities as many pharmaceutical companies redeployed their MSL teams and moved them away from commercially aligned departments like sales and marketing. As a result, most MSL teams became part of separate Medical Affairs departments which helped solidify the creation of an unbiased resource for KOLs and heavily influenced the primary activity and focus for most MSL teams.
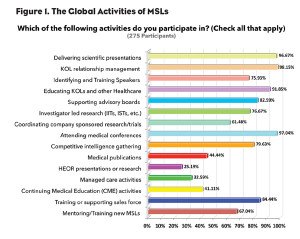
To gain insight into the range of activities MSLs are involved with and to discover if they have a primary focus regardless of where they might be located, the Medical Science Liaison Society conducted a global survey last month that included 275 MSLs from 29 countries. Among other questions in the survey, MSLs were given a list of activities and asked to select all those they are involved with.
Interestingly, according to the results, even without any guidelines or standards that specifically address the MSL role, it is clear there are some unwritten global standards and focus for MSL activities. Although MSLs are involved in multiple activities, regardless of which country the MSL works in, the vast majority of these activities are identical and primarily revolve around engaging with KOLs. In fact, over 98% of MSLs reported that they were responsible for KOL relationship management and around 92% reported educating KOLs (see Figure 1).
As the MSL role increasingly becomes recognized as a crucial component to the success of companies, it will be necessary to have guidelines that specifically address the MSL role, their training and activities.
Additional results of the survey and MSL training standards will be shared and discussed in future publications as well as at the upcoming MSL Society conference in Paris, October 28th to 30th.