TeleMed Text: First Wearable Health Record Arrives
The medical electronics start-up, Drchrono, has developed a new app for Google Glass that they call the first “wearable health record.” Doctors can register for the Drchrono app and then record every step of the diagnostic and treatment process, with the patient’s permission. The app can record photos, notes and even videos of surgeries. The data is then stored in the electronic medical record technology or in Box, a cloud-based storage and collaboration service. To develop the technology, Drchrono worked with Box, as well as the Google Glass team.
Dr. Bill J. Metaxas, a podiatrist from San Francisco, claimed that 99% of his patients consented to the use of the technology, but most physicians had not yet taken advantage of it—possibly because it is no more or less secure than the iPad. There are also several other concerns, such as obtaining patient consent for every use of the technology and difficulties with the security settings.
“Google is still in the early stages of determining the most viable use cases for Google Glass,” said Drchrono co-founder Daniel Kivatinos in a statement. “But some doctors are demanding Glass, so Google is providing resources and support to developers.”
Drchrono claims that 60,000 physicians have registered with their app and that more than 300 have already begun to use it. It is currently available for free, but there may be a fee for the service in the future.
Doctor Docs: One Third of Physicians Recommend Health Apps
According to the latest Manhattan Research Taking the Pulse survey, which polled 3,066 practicing physicians, over a third of doctors in the United States recommended a health app to patients in the past year. This piece of data, as well as several others from the survey, indicated that physician usage of mobile and digital health tools is steadily increasing.
For instance, the survey reports that 40% of physicians increased their usage of digital tools. Of the 47% with smartphones, physicians said they used them to show their patients images or videos. Also, 40% felt that increase in the usage of digital technology for patient care would improve patient outcomes.
The survey also suggested that this trend is the result of a shift towards outcomes-based care models; it shows that physicians are increasingly evaluated according to cost of treatment, patient outcomes or referrals.
“As we move to an outcomes-based model of healthcare provision in the U.S., remote monitoring and telehealth are going to drive an extension of the point-of-care,” Director of Physician Research James Avallone said in a statement. “We’re seeing physician attitudes really align with policy.”
In addition to the general increase in the usage of digital technology, in the past year more than 20% of physicians monitored their patients remotely, and those that did monitored an average of 22 patients per month.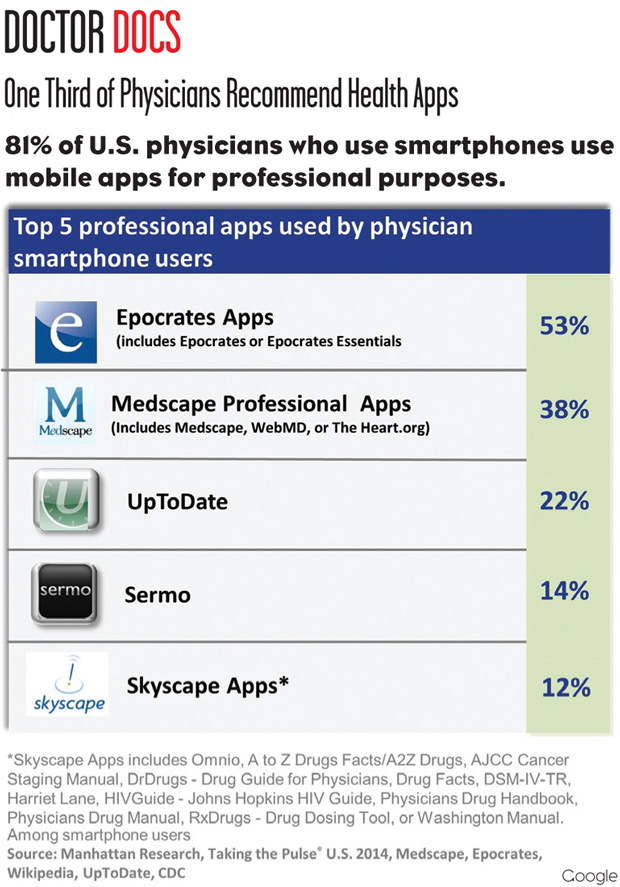
Discoveries/Innovations: Test Uncovers Cancer Genes, Best Treatment
A new genetic test may help cancer patients both identify the genes that are contributing to the cause of their cancer and provide insight into the best avenue for treatment. This more comprehensive test is now in clinical trials, and it has the potential to allow doctors to recommend the correct drug earlier in the treatment process.
If found to be more efficient, this test may replace the current tests, called companion diagnostics, which only look for one type of biological marker that predicts whether targeted therapies will be effective in treating a tumor. Dr Richard Pazdur, the U.S. Food and Drug Administration’s oncology chief, said in a statement “We really are moving away from this—one drug, one biomarker, one companion diagnostic.”
Medical Devices/Diagnostics: DNA Test Quickly Diagnoses Rare Infection
Using DNA testing, doctors were able to come to a more timely diagnosis of a rare bacterial infection in a young boy with encephalitis. The use of the groundbreaking diagnostic method, which involves the extraction of the patient’s cerebrospinal fluid, may become routine, particularly in cases in which physicians are pressed for time.
The method allowed the doctors of Family Children’s Hospital in Madison, Wisconsin (working with researchers from UC San Francisco) to diagnose the boy within 48 hours. Dr. Joseph DeRisi, a biochemist at UCSF, said in a statement, “It’s a demonstration that this technology has arrived—it can make a difference in real time.”
FDA Update
openFDA Initiative
The FDA is opening their adverse-event data using an application programming interface (API). Previously, biopharma companies needed to send Freedom of Information Act requests in order to access the information, but with the first step of the openFDA initiative, the red tape has been cut down considerably. Software developers can make the information more readily available to both patients and researchers by using the API with their app or website.
FDA Approvals
The FDA has recently approved BioDelivery’s drug Bunavail, which is intended to treat opioid dependence. Itamar Medical also received approval for their upgraded model of WatchPAT Unified, a watch-like device with diagnostic capabilities meant to diagnose sleep-breathing disorders such as sleep apnea. The FDA also approved a priority review for Imbruvica, the new drug from Pharmacyclics, Inc. to treat chronic lymphocytic leukemia (CLL) as well as small lymphocytic lymphoma (SLL). Patients who have had at least one prior therapy will receive a review for this treatment. Biogen Idec also obtained FDA approval for their new drug Eloctate, an experimental therapy that treats various symptoms of haemophilia A, such as bleeding episodes and routine prophylaxis.
FDA Warnings
HeartWare International Inc., a corporation that makes small implantable heart pumps for patients suffering from advanced heart failure, released a statement that they had received a warning letter from the FDA. The letter came in response to a manufacturing plant inspection in January. To date, the use of HeartWare devices has not been restricted.





